当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unexpected substituent’s effects on catalytic activity in the ring-opening polymerization of ε-CL and δ-VL catalyzed by β-pyridyl-enamino Al complexes
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.eurpolymj.2020.109626 Lu Qin , Fangqin Cheng , Moris S. Eisen , Xia Chen
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.eurpolymj.2020.109626 Lu Qin , Fangqin Cheng , Moris S. Eisen , Xia Chen
|
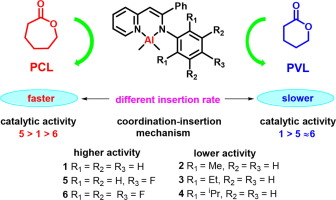
Abstract This contribution illustrates the significant influence of the Al-catalyzed e-caprolactone (e-CL) and δ-valerolactone (δ-VL) ring-opening polymerizations. A series of β-pyridyl-enamino aluminum complexes [AlLMe2] 1–6 {L = [(2-C5H4N)-C(H) C(Ph) N(Ar) ], Ar = C6H5 (1), 2,6-Me2C6H3 (2), 2,6-Et2C6H3 (3), 2,6-iPr2C6H3 (4), 4-FC6H4 (5), C6F5 (6)} were synthesized and characterized by NMR spectroscopy and single-crystal X-ray diffraction. The catalytic activities the polymerization of e-caprolactone (e-CL) and δ-valerolactone (δ-VL) correlated with the substituent’s character of the ortho and para groups on the Ar moieties of the β-pyridyl-enamino ligands. For the polymerizations of the both cyclic esters, complexes 1, 5 and 6 displayed excellent catalytic activities in the presence of the initiator benzyl alcohol, while complexes 2–4 exhibited much lower activities under the same conditions. Kinetic studies revealed that complex 5 polymerized e-CL at a faster rate in comparison to 1 and 6, where highlighted a cumulative effect of both the electronic and steric effect on the ligands. By contrast, for the ROP of δ-VL, complex 1 was found to be more active than the catalysts 5 and 6. This unusual result was suggested the rate-determined step was the “insertion” step in e-CL and δ-VL polymerization, the former’s insertion rate is faster than the latter’s.
中文翻译:

β-吡啶基-烯氨基铝配合物催化ε-CL和δ-VL开环聚合反应中意外取代基对催化活性的影响
摘要 这一贡献说明了铝催化的ε-己内酯(e-CL)和δ-戊内酯(δ-VL)开环聚合的显着影响。一系列β-吡啶基-烯氨基铝配合物[AlLMe2] 1-6 {L = [(2-C5H4N)-C(H) C(Ph) N(Ar) ], Ar = C6H5 (1), 2,6 -Me2C6H3 (2), 2,6-Et2C6H3 (3), 2,6-iPr2C6H3 (4), 4-FC6H4 (5), C6F5 (6)} 合成并通过核磁共振光谱和单晶X射线表征衍射。ε-己内酯(e-CL)和δ-戊内酯(δ-VL)的聚合催化活性与β-吡啶基-烯氨基配体的Ar部分上的邻位和对位基团的取代基特征相关。对于两种环酯的聚合,配合物 1、5 和 6 在引发剂苯甲醇存在下表现出优异的催化活性,而在相同条件下,复合物 2-4 的活性要低得多。动力学研究表明,与 1 和 6 相比,复合物 5 以更快的速率聚合 e-CL,其中突出了电子和空间效应对配体的累积效应。相比之下,对于 δ-VL 的 ROP,发现配合物 1 比催化剂 5 和 6 更具活性。这一不寻常的结果表明速率决定步骤是 e-CL 和 δ-VL 中的“插入”步骤聚合时,前者的插入速度比后者快。
更新日期:2020-04-01
中文翻译:

β-吡啶基-烯氨基铝配合物催化ε-CL和δ-VL开环聚合反应中意外取代基对催化活性的影响
摘要 这一贡献说明了铝催化的ε-己内酯(e-CL)和δ-戊内酯(δ-VL)开环聚合的显着影响。一系列β-吡啶基-烯氨基铝配合物[AlLMe2] 1-6 {L = [(2-C5H4N)-C(H) C(Ph) N(Ar) ], Ar = C6H5 (1), 2,6 -Me2C6H3 (2), 2,6-Et2C6H3 (3), 2,6-iPr2C6H3 (4), 4-FC6H4 (5), C6F5 (6)} 合成并通过核磁共振光谱和单晶X射线表征衍射。ε-己内酯(e-CL)和δ-戊内酯(δ-VL)的聚合催化活性与β-吡啶基-烯氨基配体的Ar部分上的邻位和对位基团的取代基特征相关。对于两种环酯的聚合,配合物 1、5 和 6 在引发剂苯甲醇存在下表现出优异的催化活性,而在相同条件下,复合物 2-4 的活性要低得多。动力学研究表明,与 1 和 6 相比,复合物 5 以更快的速率聚合 e-CL,其中突出了电子和空间效应对配体的累积效应。相比之下,对于 δ-VL 的 ROP,发现配合物 1 比催化剂 5 和 6 更具活性。这一不寻常的结果表明速率决定步骤是 e-CL 和 δ-VL 中的“插入”步骤聚合时,前者的插入速度比后者快。











































 京公网安备 11010802027423号
京公网安备 11010802027423号