Journal of Proteomics ( IF 2.8 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jprot.2020.103734 Virgínia Campos Silvestrini 1 , Carolina Hassibe Thomé 1 , Daniele Albuquerque 1 , Camila de Souza Palma 1 , Germano Aguiar Ferreira 1 , Guilherme Pauperio Lanfredi 2 , Ana Paula Masson 2 , Lara Elis Alberici Delsin 1 , Fernanda Ursoli Ferreira 3 , Felipe Canto de Souza 3 , Lyris Martins Franco de Godoy 4 , Adriano Aquino 5 , Emanuel Carrilho 5 , Rodrigo Alexandre Panepucci 3 , Dimas Tadeu Covas 3 , Vitor Marcel Faça 1
|
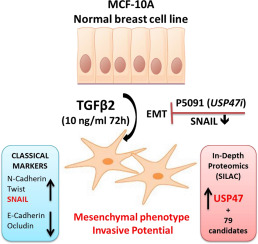
Epithelial to Mesenchymal Transition (EMT) is a normal cellular process that is also triggered during cancer progression and metastasis. EMT induces cellular and microenviromental changes, resulting in loss of epithelial features and acquisition of mesenchymal phenotypes. The growth factor TGFβ and the transcription factor SNAIL1 (SNAIL) have been described as inducers of EMT. Here, we carried out an EMT model with non-tumorigenic cell line MCF-10A induced with the TGFβ2 specific isoform of TGF protein family. The model was validated by molecular, morphological and functional experiments and showed correlation with the up-regulation of SNAIL. In order to identify additional regulators of EMT in this non-tumorigenic model, we explored quantitative proteomics, which revealed the Ubiquitin carboxyl-terminal hydrolase 47 (USP47) as one of the top up-regulated proteins. USP47 has a known role in cell growth and genome integrity, but not previously correlated to EMT. After validating USP47 alterations using MRM and antibody-based assays, we demonstrated that the chemical inhibition of USP47 with the inhibitor P5091 reduced expression of EMT markers and reverted morphological changes in MCF-10A cells undergoing EMT. These results support the involvement of USP47 in our EMT model as well as potential applications of deubiquitinases as therapeutic targets for cancer progression management.
Biological significance:
Metastasis is responsible for most cancer-associated mortality. Additionally, metastasis requires special attention, as the cellular transformations make treatment at this stage very difficult or occasionally impossible. Early steps in cancer metastasis involve the ability to detach from the solid tumor mass and invade the surrounding stromal tissues through cohesive migration, or a mesenchymal or amoeboid invasion. One of the first steps for metastatic cascade is denominated epithelial to mesenchymal transition (EMT), which can be triggered by different factors. Here, our efforts were directed to better understand this process and identify new pathways that contributes for acquisition to EMT, mainly related to post translational modifications related to ubiquitin proteasome system. Our model of EMT induction by TGFβ2 mimics early stage of metastatic cancer in epithelial breast cells and a proteomic study carried out for such model demonstrates that the deubiquitinase enzyme USP47 acts in SNAIL stabilization, one of the most important transcription factors for EMT phenotype acquisition and consequent metastasis. In addition, the inhibiton of USP47 with P5091, reverted the EMT phenotype. Together the knowledge of such processes of cancer progression and regulation can help in designing new strategies for combined therapies for control of cancer in early stages.
中文翻译:

蛋白质组学分析揭示了泛素特异性蛋白酶 (USP47) 在 TGFβ2 诱导乳腺细胞上皮间质转化 (EMT) 中的作用
上皮间质转化 (EMT) 是一种正常的细胞过程,在癌症进展和转移过程中也会触发。EMT 诱导细胞和微环境变化,导致上皮特征的丧失和间充质表型的获得。生长因子 TGFβ 和转录因子 SNAIL1 (SNAIL) 已被描述为 EMT 的诱导剂。在这里,我们用 TGF 蛋白家族的 TGFβ2 特异性同种型诱导的非致瘤细胞系 MCF-10A 进行了 EMT 模型。该模型通过分子、形态学和功能实验验证,并显示出与 SNAIL 上调的相关性。为了在这种非致瘤模型中识别 EMT 的其他调节因子,我们探索了定量蛋白质组学,这揭示了泛素羧基末端水解酶 47 (USP47) 作为最高上调的蛋白质之一。USP47 在细胞生长和基因组完整性中具有已知作用,但以前与 EMT 无关。在使用 MRM 和基于抗体的测定验证 USP47 改变后,我们证明了用抑制剂 P5091 对 USP47 的化学抑制降低了 EMT 标志物的表达,并恢复了经历 EMT 的 MCF-10A 细胞的形态变化。这些结果支持 USP47 参与我们的 EMT 模型以及去泛素酶作为癌症进展管理的治疗靶点的潜在应用。我们证明了用抑制剂 P5091 对 USP47 的化学抑制降低了 EMT 标志物的表达,并恢复了经历 EMT 的 MCF-10A 细胞的形态变化。这些结果支持 USP47 参与我们的 EMT 模型以及去泛素酶作为癌症进展管理的治疗靶点的潜在应用。我们证明了用抑制剂 P5091 对 USP47 的化学抑制降低了 EMT 标志物的表达,并恢复了经历 EMT 的 MCF-10A 细胞的形态变化。这些结果支持 USP47 参与我们的 EMT 模型以及去泛素酶作为癌症进展管理的治疗靶点的潜在应用。
生物学意义:
转移是造成大多数癌症相关死亡率的原因。此外,转移需要特别注意,因为细胞转化使此阶段的治疗非常困难或有时是不可能的。癌症转移的早期步骤涉及从实体瘤块脱离并通过内聚性迁移或间充质或变形虫侵入侵入周围基质组织的能力。转移级联的第一步被称为上皮间质转化 (EMT),它可以由不同的因素触发。在这里,我们的努力旨在更好地理解这一过程并确定有助于获得 EMT 的新途径,主要与泛素蛋白酶体系统相关的翻译后修饰有关。我们的 TGFβ2 诱导 EMT 模型模拟了上皮乳腺细胞中转移性癌症的早期阶段,对这种模型进行的蛋白质组学研究表明,去泛素酶 USP47 在 SNAIL 稳定中起作用,这是 EMT 表型获得和随后的最重要的转录因子之一转移。此外,USP47 与 P5091 的抑制作用恢复了 EMT 表型。对这些癌症进展和调节过程的了解有助于设计用于早期控制癌症的联合疗法的新策略。此外,USP47 与 P5091 的抑制作用恢复了 EMT 表型。对这些癌症进展和调节过程的了解有助于设计用于早期控制癌症的联合疗法的新策略。此外,USP47 与 P5091 的抑制作用恢复了 EMT 表型。对这些癌症进展和调节过程的了解有助于设计用于早期控制癌症的联合疗法的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号