Journal of Proteomics ( IF 2.8 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jprot.2020.103735 Cher P Ooi 1 , Corinna Benz 2 , Michael D Urbaniak 2
|
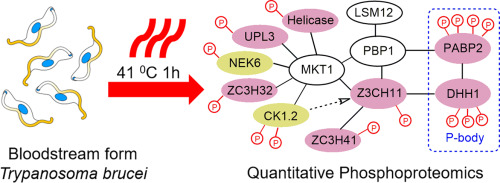
The symptoms of African sleeping sickness, caused by the parasite Trypanosoma brucei, can include periods of fever as high as 41 °C which triggers a heat shock response in the parasite. To capture events involved in sensing and responding to heat shock in the mammalian infective form we have conducted a SILAC-based quantitative proteomic and phosphoproteomic analysis of T. brucei cells treated at 41 °C for 1h. Our analysis identified 193 heat shock responsive phosphorylation sites with an average of 5-fold change in abundance, but only 20 heat shock responsive proteins with average of 1.5-fold change. These data indicate that protein abundance does not rapidly respond (≤1 h) to heat shock, and that the changes observed in phosphorylation site abundance are larger and more widespread. The heat shock responsive phosphorylation sites showed enrichment of RNA binding proteins with putative roles in heat shock response included P-body / stress granules and the eukaryotic translation initiation 4F complex. The ZC3H11-MKT1 complex, which stabilises mRNAs of thermotolerance proteins, appears to represent a key signal integration node in the heat shock response.
Significance
We report the first quantitative study of changes in protein and phosphorylation site abundance in response to heat shock in the clinically relevant form of the human parasite Trypanosoma brucei. The identification of heat shock responsive phosphorylation sites on proteins with putative roles in thermotolerance including the ZC3H11-MKT1 complex provides evidence of the role dynamic phosphorylation of RNA binding proteins in co-ordinating heat shock. Temperature changes in the host are a major physiological challenge to parasites and factors conferring tolerance to heat shock constitute overlooked virulence factors. A better understanding of these virulence factors will pave the way for the development of novel drug therapies which selectively target T. brucei.
中文翻译:

对热休克的哺乳动物感染性布氏锥虫的磷酸化蛋白质组学分析表明其耐热性的非典型机制
非洲昏睡病是由寄生虫布氏锥虫引起的,其症状可能包括高达 41 ° C 的发烧,从而引发寄生虫的热休克反应。为了捕获哺乳动物感染形式中与热休克的感知和响应有关的事件,我们对在 41 ° C处理 1 小时的布氏锥虫细胞进行了基于 SILAC 的定量蛋白质组和磷酸蛋白质组分析。我们的分析确定了 193 个热休克响应性磷酸化位点,平均丰度变化为 5 倍,但只有 20 个热休克响应蛋白平均丰度变化为 1.5 倍。这些数据表明蛋白质丰度对热休克不会快速响应(≤1小时),并且观察到的磷酸化位点丰度的变化更大、更广泛。热休克反应性磷酸化位点显示出在热休克反应中具有推定作用的RNA结合蛋白的富集,包括P体/应激颗粒和真核翻译起始4F复合物。ZC3H11-MKT1 复合物可稳定耐热蛋白的 mRNA,似乎代表热激反应中的关键信号整合节点。
意义
我们报告了第一个定量研究,研究了人类寄生虫布氏锥虫对热休克的反应,蛋白质和磷酸化位点丰度的变化。包括 ZC3H11-MKT1 复合物在内的在耐热性中具有推定作用的蛋白质上热休克响应性磷酸化位点的鉴定提供了 RNA 结合蛋白动态磷酸化在协调热休克中的作用的证据。宿主的温度变化是寄生虫面临的主要生理挑战,而赋予热激耐受性的因素构成了被忽视的毒力因素。更好地了解这些毒力因子将为开发选择性靶向布氏锥虫的新型药物疗法铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号