Catalysis Today ( IF 5.2 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.cattod.2020.03.036 Vladimir Paunović , Marcos Rellán-Piñeiro , Núria López , Javier Pérez-Ramírez
|
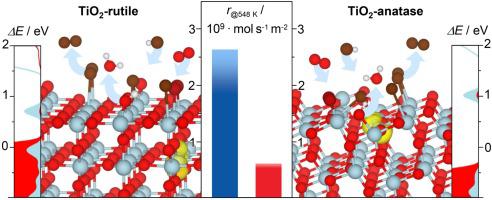
This article investigates the activity of TiO2-rutile and TiO2-anatase polymorphs in the catalytic HBr oxidation, which is an enabling process to close the halogen loop in bromine-mediated transformation of natural gas to high-value chemicals and liquid fuels. The evaluation of rutile-, anatase-, and rutile-anatase TiO2 catalysts, exhibiting the variable specific surface areas, revealed that anatase phase is also active in this reaction. Nonetheless, in contrast to photocatalytic processes in which anatase is typically more active than rutile, rutile exhibits ca. 2–5 times higher intrinsic rates of HBr oxidation than anatase. Thereby, the apparent activation energies and reaction orders with respect to HBr, O2, and H2O display similar values for the two polymorphs. The activity differences were rationalized by density functional theory analysis, which showed that HBr oxidation follows a similar defect-driven mechanism over the most stable rutile (110) and anatase (101) surfaces. Herein, HBr activates the catalyst through a self-doping mechanism that involves the substitution of surface oxygen by bromine with the concomitant reduction of Ti4+ to Ti3+ centers. This forms a defect level that is placed in the band gap and allows for the O2 activation on the catalyst surface. While the HBr adsorption and H2O desorption display a similar energy profiles on both polymorphs, the O2 activation and Br2 evolution are more facile over rutile compared to anatase surface due to shorter distances between the coordinatively unsaturated Ticus sites and easier reduction of Ti4+ centers upon product desorption, respectively.
中文翻译:

金红石型和锐钛矿型TiO 2多晶型在HBr催化中的活性差异
本文研究了TiO 2金红石型和TiO 2锐钛矿型多晶型物在催化HBr氧化中的活性,这是在溴介导的天然气向高价值化学品和液体燃料的转化中封闭卤素环的一个使能过程。表现出可变的比表面积的金红石型,锐钛矿型和金红石型锐钛矿型TiO 2催化剂的评估表明,锐钛矿相在该反应中也具有活性。尽管如此,与锐钛矿通常比金红石活性更高的光催化过程相反,金红石表现出约10%的活性。HBr氧化的固有速率是锐钛矿的2–5倍。由此,关于HBr,O 2的表观活化能和反应阶数和,H 2 O对这两个多晶型物显示相似的值。通过密度泛函理论分析使活性差异合理化,结果表明,在最稳定的金红石(110)和锐钛矿(101)表面上,HBr氧化遵循相似的缺陷驱动机制。在此,HBr通过自掺杂机制活化催化剂,该自掺杂机制包括用溴取代表面氧以及伴随的Ti 4+到Ti 3+中心的还原。这形成了位于带隙中的缺陷水平,并允许催化剂表面上的O 2活化。虽然HBr吸附和H 2 O解吸在两种多晶型物上都显示出相似的能量分布,但O 2与锐钛矿表面相比,活化和Br 2的析出在金红石上更容易,这是由于配位不饱和Ti cus位点之间的距离更短,并且在产物解吸时Ti 4+中心更容易还原。









































 京公网安备 11010802027423号
京公网安备 11010802027423号