Journal of the Energy Institute ( IF 5.6 ) Pub Date : 2020-02-26 , DOI: 10.1016/j.joei.2020.02.005 Qing Xu , Lijun Zhang , Kai Sun , Yuewen Shao , Hongli Tian , Shu Zhang , Qing Liu , Guangzhi Hu , Shuang Wang , Xun Hu
|
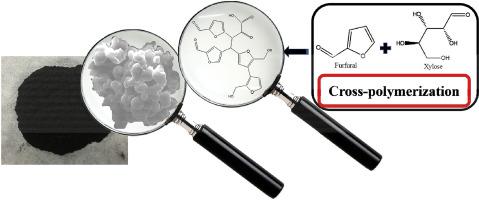
Understanding the potential cross-polymerisation between the main components in bio-oil is essential to develop the method to minimize the coking in hydrotreatment of bio-oil or to maximize the production of carbon material from bio-oil. In this study, the potential cross-polymerisation between furfural and the sugars (xylose and glucose) and the impacts of acid/base catalysts on the polymerisation reactions were investigated. The results indicated that the cross-polymerisation of furfural/xylose and furfural/glucose existed in absence of catalyst, and the extent was more significant for that between furfural and glucose. The strong acid promoted the cross-polymerisation while the weak organic acid like acetic acid and the strong base like NaOH did not. The cross-polymerisation of furfural/sugars produced the more hydrophilic carbonaceous spheres that tended to merge to form bigger particles. The polymerisation of furfural involved opening of the furan ring via hydrolysis, forming oxygen-containing intermediates, while glucose/xylose were more reactive towards polymerisation reaction via dehydration route. The polymers from the sugars were more aliphatic than that from furfural, especially for that from glucose, leading to their lower thermal stabilities. The cross-polymerisation also affected the size/abundance of the π-conjugated structures in the soluble polymers and the relative ratio of the C O/C
O/C C in the resulting insoluble polymer.
C in the resulting insoluble polymer.
中文翻译:

模型呋喃与生物油中的碳水化合物与酸或碱催化剂的交叉聚合
了解生物油中主要成分之间潜在的交叉聚合对于开发该方法以最小化生物油加氢处理中的焦化或最大程度地利用生物油生产碳材料至关重要。在这项研究中,研究了糠醛和糖(木糖和葡萄糖)之间潜在的交叉聚合以及酸/碱催化剂对聚合反应的影响。结果表明,糠醛/木糖与糠醛/葡萄糖的交叉聚合在不存在催化剂的情况下存在,其程度在糠醛与葡萄糖之间更为明显。强酸促进了交叉聚合,而弱有机酸(如乙酸)和强碱(如NaOH)则没有。糠醛/糖的交叉聚合产生了亲水性更高的碳球,这些碳球趋于合并形成更大的颗粒。糠醛的聚合涉及通过水解打开呋喃环,形成含氧中间体,而葡萄糖/木糖通过脱水途径对聚合反应更具反应性。来自糖的聚合物比来自糠醛的聚合物具有更高的脂肪含量,尤其是来自葡萄糖的聚合物,从而导致其较低的热稳定性。交叉聚合还影响可溶性聚合物中π共轭结构的大小/丰度以及C的相对比例 葡萄糖/木糖通过脱水途径对聚合反应更具反应性。来自糖的聚合物比来自糠醛的聚合物具有更高的脂肪含量,尤其是来自葡萄糖的聚合物,从而导致其较低的热稳定性。交叉聚合还影响可溶性聚合物中π共轭结构的大小/丰度以及C的相对比例 葡萄糖/木糖通过脱水途径对聚合反应更具反应性。来自糖的聚合物比来自糠醛的聚合物具有更高的脂肪含量,尤其是来自葡萄糖的聚合物,从而导致其较低的热稳定性。交叉聚合还影响可溶性聚合物中π共轭结构的大小/丰度以及C的相对比例
 所得不溶聚合物中的O / C C。
所得不溶聚合物中的O / C C。











































 京公网安备 11010802027423号
京公网安备 11010802027423号