当前位置:
X-MOL 学术
›
J. Insect Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two insulin receptors coordinate oogenesis and oviposition via two pathways in the green lacewing, Chrysopa pallens.
Journal of Insect Physiology ( IF 2.3 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.jinsphys.2020.104049 Benfeng Han 1 , Tingting Zhang 2 , Yanjiao Feng 1 , Xiaopin Liu 1 , Lisheng Zhang 1 , Hongyin Chen 1 , Fanrong Zeng 1 , Mengqing Wang 1 , Chenxi Liu 1 , Yuyan Li 1 , Jinjie Cui 3 , Zhaoqun Li 4 , Jianjun Mao 1
Journal of Insect Physiology ( IF 2.3 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.jinsphys.2020.104049 Benfeng Han 1 , Tingting Zhang 2 , Yanjiao Feng 1 , Xiaopin Liu 1 , Lisheng Zhang 1 , Hongyin Chen 1 , Fanrong Zeng 1 , Mengqing Wang 1 , Chenxi Liu 1 , Yuyan Li 1 , Jinjie Cui 3 , Zhaoqun Li 4 , Jianjun Mao 1
Affiliation
|
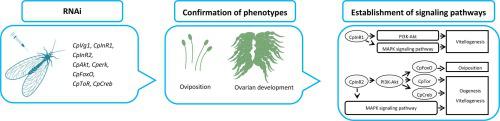
Insulin signalling in insects, as in mammals, regulates various physiological functions, such as reproduction. However, the molecular mechanism by which insulin signals orchestrate ovarian stem cell proliferation, vitellogenesis, and oviposition remains elusive. Here, we investigate the functions of the phosphoinositide 3-kinase (PI3K)-serine/threonine kinase (Akt) pathway, GTPase Ras/mitogen-activated protein kinase (MAPK) pathway, and their downstream messengers in a natural predator, Chrysopa pallens, by the RNAi method. When C. pallens vitellogenin gene 1 (CpVg1) expression was knocked down, the follicle maturation was arrested and total fecundity was reduced. Silencing C. pallens insulin receptor 1 (CpInR1) suppressed Vg transcription and reduced egg mass and hatching rate. Depletion of C. pallens insulin receptor 2 (CpInR2) transcripts lowered Vg transcript level, hampered ovarian development and decreased reproductive output. Knockdown of C. pallens Akt (CpAkt) and C. pallens extracellular-signal-regulated kinase (Cperk) caused phenotypes similar to those caused by knockdown of CpInR2. Disruption of C. pallens transcription factor forkhead box O (CpFoxO) expression caused no significant effects on ovarian development, but sharply impaired total fecundity. Interference with the expression of C. pallens target of rapamycin (CpTor) gene and C. pallens cAMP-response element binding protein (CpCreb) gene led to a down-regulation of Vg transcription, blocking of ovariole growth, and decrease in egg quality. These results suggested the two CpInRs orchestrate oogenesis and oviposition via two signalling pathways to guarantee natural reproduction in the green lacewing, C. pallens.
中文翻译:

两个胰岛素受体通过绿色草lace(Chrysopa pallens)中的两个途径协调卵子形成和产卵。
像哺乳动物一样,昆虫中的胰岛素信号会调节各种生理功能,例如生殖。但是,胰岛素信号调控卵巢干细胞增殖,卵黄发生和产卵的分子机制仍然难以捉摸。在这里,我们研究了磷酸肌醇3激酶(PI3K)-丝氨酸/苏氨酸激酶(Akt)途径,GTPase Ras /丝裂原激活的蛋白激酶(MAPK)途径及其在天然捕食者Chrysopa pallens中的下游信使的功能,通过RNAi方法。当pallens C.卵黄蛋白原基因1(CpVg1)的表达被敲低时,卵泡的成熟被停止,总生殖力降低。沉默帕氏梭菌胰岛素受体1(CpInR1)可抑制Vg转录并降低卵量和孵化率。C的耗尽 Pallens胰岛素受体2(CpInR2)转录本可降低Vg转录本水平,阻碍卵巢发育并降低生殖输出。Pallens Akt(CpAkt)和Pallens C.pallens细胞外信号调节激酶(Cperk)的敲除引起的表型类似于CpInR2的敲除引起的表型。淡色梭菌转录因子叉头盒O(CpFoxO)表达的破坏对卵巢发育没有明显影响,但严重损害了总生殖力。干扰雷帕霉素靶蛋白(CpTor)基因和鲍氏cAMP反应元件结合蛋白(CpCreb)基因的表达导致Vg转录下调,卵巢生长受阻并降低鸡蛋品质。
更新日期:2020-04-21
中文翻译:

两个胰岛素受体通过绿色草lace(Chrysopa pallens)中的两个途径协调卵子形成和产卵。
像哺乳动物一样,昆虫中的胰岛素信号会调节各种生理功能,例如生殖。但是,胰岛素信号调控卵巢干细胞增殖,卵黄发生和产卵的分子机制仍然难以捉摸。在这里,我们研究了磷酸肌醇3激酶(PI3K)-丝氨酸/苏氨酸激酶(Akt)途径,GTPase Ras /丝裂原激活的蛋白激酶(MAPK)途径及其在天然捕食者Chrysopa pallens中的下游信使的功能,通过RNAi方法。当pallens C.卵黄蛋白原基因1(CpVg1)的表达被敲低时,卵泡的成熟被停止,总生殖力降低。沉默帕氏梭菌胰岛素受体1(CpInR1)可抑制Vg转录并降低卵量和孵化率。C的耗尽 Pallens胰岛素受体2(CpInR2)转录本可降低Vg转录本水平,阻碍卵巢发育并降低生殖输出。Pallens Akt(CpAkt)和Pallens C.pallens细胞外信号调节激酶(Cperk)的敲除引起的表型类似于CpInR2的敲除引起的表型。淡色梭菌转录因子叉头盒O(CpFoxO)表达的破坏对卵巢发育没有明显影响,但严重损害了总生殖力。干扰雷帕霉素靶蛋白(CpTor)基因和鲍氏cAMP反应元件结合蛋白(CpCreb)基因的表达导致Vg转录下调,卵巢生长受阻并降低鸡蛋品质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号