当前位置:
X-MOL 学术
›
Regul. Toxicol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acute and repeated dose 28-day oral toxicity of Chrysobalanusicaco L. leaf aqueous extract.
Regulatory Toxicology and Pharmacology ( IF 3.0 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.yrtph.2020.104643 Natalie Emanuelle Ribeiro 1 , Pedro Silvino Pereira 1 , Tatiane Bezerra de Oliveira 1 , Sandrine Maria de Arruda Lima 1 , Tania Maria Sarmento Silva 2 , Andréa Lopes Bandeira Delmiro Santana 1 , Márcia Silva do Nascimento 1 , Rogelio Moreno Santisteban 2 , Álvaro Aguiar Coelho Teixeira 3 , Teresinha Gonçalves da Silva 1
Regulatory Toxicology and Pharmacology ( IF 3.0 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.yrtph.2020.104643 Natalie Emanuelle Ribeiro 1 , Pedro Silvino Pereira 1 , Tatiane Bezerra de Oliveira 1 , Sandrine Maria de Arruda Lima 1 , Tania Maria Sarmento Silva 2 , Andréa Lopes Bandeira Delmiro Santana 1 , Márcia Silva do Nascimento 1 , Rogelio Moreno Santisteban 2 , Álvaro Aguiar Coelho Teixeira 3 , Teresinha Gonçalves da Silva 1
Affiliation
|
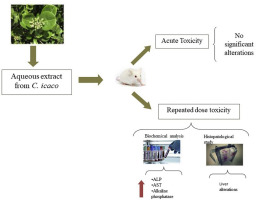
Chrysobalanus icaco L. is a native plant of Brazil used as a food source and traditionally for the treatment of various diseases. The aim of study was performed the phytochemical analysis by UPLC-DAD-ESI-QTOF-MS/MS, and evaluated acute and repeated dose oral toxicities of the C. icaco L. leaf aqueous extract (AECi). The acute toxicity study was performed using a dose of AECi 2000 mg/kg, while the repeated dose toxicity study, the AECi was administered daily at doses of 100, 200 and 400 mg/kg, for 28 days. Behavior and mortality of animals were observed during the test period and body weight, as well water and eating consumption. Hematological, biochemical parameters and histopathological examinations were carried out. Phytochemical analysis of the AECi revealed the presence of flavonoids and tannins. Oral single dose of 2000 mg/kg of AECi resulted in no mortalities or abnormal clinical signs. Studies of repeated dose toxicity promoted a reduction in the body weight of treated animals and an increase of hepatic enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in both, males and females. Histopathological analyzes showed alterations in the livers of animals treated with AECi. Thus, this study recommends the population take care when using this species, especially during prolonged periods.
中文翻译:

Chrysobalanusicaco L.叶水提取物的急性和重复剂量28天口服毒性。
Chrysobalanus icaco L.是巴西的一种本地植物,用作食物来源,传统上用于治疗各种疾病。研究的目的是通过UPLC-DAD-ESI-QTOF-MS / MS进行植物化学分析,并评估C. icaco L.叶水提取物(AECi)的急性和重复剂量口服毒性。急性毒性研究使用2000 mg / kg的AECi剂量进行,而重复剂量毒性研究则每天以100、200和400 mg / kg的剂量施用AECi,持续28天。在测试期间和体重,水和饮食消耗中观察到动物的行为和死亡率。进行了血液学,生化参数和组织病理学检查。AECi的植物化学分析表明,类黄酮和丹宁酸的存在。口服单剂量2000 mg / kg的AECi不会导致死亡或异常的临床体征。重复剂量毒性的研究促进了雄性和雌性动物体重减轻和肝酶丙氨酸氨基转移酶(ALT)和天冬氨酸氨基转移酶(AST)的增加。组织病理学分析显示,用AECi处理的动物的肝脏发生了变化。因此,本研究建议人群在使用该物种时应格外小心,尤其是在长时间内。组织病理学分析显示,用AECi处理的动物的肝脏发生了变化。因此,这项研究建议人群在使用该物种时应格外小心,尤其是在长时间内。组织病理学分析显示,用AECi处理的动物的肝脏发生了变化。因此,这项研究建议人群在使用该物种时应格外小心,尤其是在长时间内。
更新日期:2020-03-20
中文翻译:

Chrysobalanusicaco L.叶水提取物的急性和重复剂量28天口服毒性。
Chrysobalanus icaco L.是巴西的一种本地植物,用作食物来源,传统上用于治疗各种疾病。研究的目的是通过UPLC-DAD-ESI-QTOF-MS / MS进行植物化学分析,并评估C. icaco L.叶水提取物(AECi)的急性和重复剂量口服毒性。急性毒性研究使用2000 mg / kg的AECi剂量进行,而重复剂量毒性研究则每天以100、200和400 mg / kg的剂量施用AECi,持续28天。在测试期间和体重,水和饮食消耗中观察到动物的行为和死亡率。进行了血液学,生化参数和组织病理学检查。AECi的植物化学分析表明,类黄酮和丹宁酸的存在。口服单剂量2000 mg / kg的AECi不会导致死亡或异常的临床体征。重复剂量毒性的研究促进了雄性和雌性动物体重减轻和肝酶丙氨酸氨基转移酶(ALT)和天冬氨酸氨基转移酶(AST)的增加。组织病理学分析显示,用AECi处理的动物的肝脏发生了变化。因此,本研究建议人群在使用该物种时应格外小心,尤其是在长时间内。组织病理学分析显示,用AECi处理的动物的肝脏发生了变化。因此,这项研究建议人群在使用该物种时应格外小心,尤其是在长时间内。组织病理学分析显示,用AECi处理的动物的肝脏发生了变化。因此,这项研究建议人群在使用该物种时应格外小心,尤其是在长时间内。











































 京公网安备 11010802027423号
京公网安备 11010802027423号