Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.comptc.2020.112793 Katarzyna Pustuła , Anna Płonka , Marcin Makowski
|
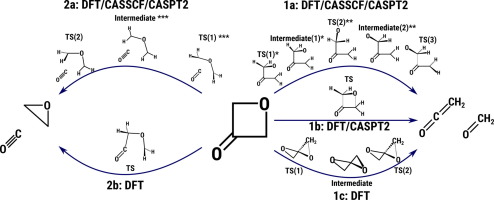
The objective of this research is to provide a theoretical explanation of the thermal decomposition of the oxetan-3-one molecule. This process may proceed via at least two distinctive reaction pathways. One of them leads to the formation of ketene and formaldehyde and the other to oxirane and carbon monoxide. We examined, on the basis of multireference approaches, the reaction profiles of oxetan-3-one pyrolysis in terms of Gibb’s free energy. The geometries and thermodynamical parameters of the transition states were obtained and compared to the known experimental facts and calculations. We also show that as some transition states have broken spin-symmetry character in DFT calculations, incorporating multireference approaches seems to be necessary. On the basis of CASPT2 modelling we were able to explain the fundamental experimental finding that the reaction leading to oxirane and carbon monoxide becomes more and more pronounced in higher temperatures and rationalized in terms of entropic contributions.
中文翻译:

多参考方法视角下的Oxetan-3-one热解
这项研究的目的是为氧杂环丁烷-3-one分子的热分解提供理论解释。该过程可以通过至少两个不同的反应途径进行。它们之一导致乙烯酮和甲醛的形成,而另一种导致环氧乙烷和一氧化碳的形成。我们基于多参考方法,根据吉布的自由能,研究了氧杂环丁烷3-one热解的反应曲线。获得过渡态的几何形状和热力学参数,并将其与已知的实验事实和计算结果进行比较。我们还表明,由于某些过渡态在DFT计算中破坏了自旋对称性,因此纳入多参考方法似乎是必要的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号