iScience ( IF 4.6 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.isci.2020.100994 Malla Reddy Gannarapu 1 , Jun Zhou 1 , Bingyao Jiang 1 , Norio Shibata 2
|
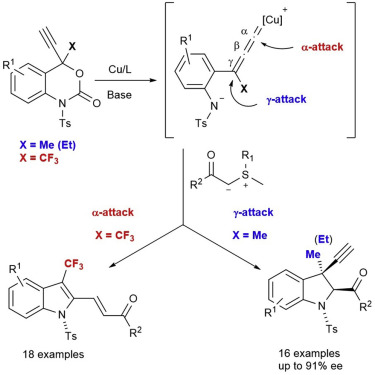
We disclose the Cu-catalyzed enantioselective synthesis of 3-methyl-3-propargyl-indolines, which contain a quaternary stereogenic carbon center, via the decarboxylative [4 + 1] annulation of 4-methyl-4-propargyl-benzoxazinanones with variety of sulfur ylides. The reaction proceeds predominantly through a γ-attack at the Cu-allenylidene intermediates by sulfur ylides to provide the corresponding indolines in good yield and high enantioselectivity (up to 91% ee). In contrast, the reaction of 4-trifluoromethyl-4-propargyl-benzoxazinanones with sulfur ylides delivers 3-trifluoromethyl-2-functionalized indoles in good to high yield via an unexpected α-attack at the Cu-allenylidene intermediates. Control over the α/γ-attack at the Cu-allenylidene intermediates by the same interceptors was achieved for the first time by the use of trifluoromethyl substituents.
中文翻译:

经由铜-亚烷基与硫叶立德的两种催化环化模式,其中存在或不存在三氟甲基取代基。
我们通过4-甲基-4-炔丙基-苯并恶嗪酮与多种硫的脱羧[4 + 1]环合,揭示了Cu催化的3-甲基-3-炔丙基-吲哚啉的对映选择性合成,该化合物含有季立体碳中心。 ylides。该反应主要通过含硫的硫化物通过在Cu-亚烯基中间体上的γ攻击进行,以提供具有良好收率和高对映选择性(最高91%ee)的相应二氢吲哚。相比之下,4-三氟甲基-4-炔丙基-苯并恶嗪酮与硫基化物的反应通过在Cu-亚烯基中间体上发生意想不到的α攻击,以高至高产率提供了3-三氟甲基-2-官能化的吲哚。










































 京公网安备 11010802027423号
京公网安备 11010802027423号