当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Including mRECIST in the Metroticket 2.0 criteria improves prediction of hepatocellular carcinoma-related death after liver transplant
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jhep.2020.03.018 Alessandro Cucchetti 1 , Matteo Serenari 2 , Carlo Sposito 3 , Stefano Di Sandro 4 , Cristina Mosconi 5 , Ilaria Vicentin 6 , Enrico Garanzini 7 , Vincenzo Mazzaferro 3 , Luciano De Carlis 8 , Rita Golfieri 9 , Carlo Spreafico 7 , Angelo Vanzulli 6 , Vincenzo Buscemi 4 , Matteo Ravaioli 2 , Giorgio Ercolani 1 , Antonio Daniele Pinna 2 , Matteo Cescon 2
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jhep.2020.03.018 Alessandro Cucchetti 1 , Matteo Serenari 2 , Carlo Sposito 3 , Stefano Di Sandro 4 , Cristina Mosconi 5 , Ilaria Vicentin 6 , Enrico Garanzini 7 , Vincenzo Mazzaferro 3 , Luciano De Carlis 8 , Rita Golfieri 9 , Carlo Spreafico 7 , Angelo Vanzulli 6 , Vincenzo Buscemi 4 , Matteo Ravaioli 2 , Giorgio Ercolani 1 , Antonio Daniele Pinna 2 , Matteo Cescon 2
Affiliation
|
BACKGROUND
S AIMS: The weight of response to neo-adjuvant therapies, to select candidates with hepatocellular carcinoma (HCC) for liver transplantation (LT) at acceptable risk of recurrence, remains partially unsolved for most of post-LT prediction models. Aim of this study was to embed radiological response in the Metroticket 2.0 model for post-LT prediction of "HCC-related death" to provide more usefulness in the modern clinical scenario. METHODS
Data from 859 transplanted patients (2000-2015) who received neo-adjuvant therapies were included. The last radiological assessment before LT was reviewed according to the mRECIST criteria. Competing-risk analysis was applied. The added value of including radiological response into the Metroticket 2.0 was explored through the category-based Net Reclassification Improvement (NRI). RESULTS
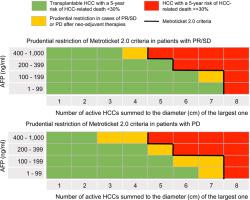
At last radiological assessment prior to LT, complete response (CR) was diagnosed in 41.3%, partial response/stable disease (PR/SD) in 24.9% and progressive disease (PD) in 33.8%. Patients with CR had 5-year rates of "HCC-related death" of 3.1%, those with PR/SD had 9.6% and those with PD had 13.4% (P<0.001). Log10AFP (p<0.001) and the sum of number and diameter of the tumour/s (p<0.05) were determinants of "HCC-related death" for PR/SD and PD patients, with different hazards. To maintain the post-LT 5-year incidence of "HCC-related death" <30%, the Metroticket 2.0 criteria were restricted in some cases of PR/SD and in all cases with PD, correctly reclassifying 9.4% of patients who died from "HCC-related death", at the expenditure of 3.5% of patients who did not have the event. The overall NRI was of 5.8. CONCLUSION
Inclusion of mRECIST criteria within the Metroticket 2.0 framework can provide further clinical information when judging eligibility for candidates to LT who received neo-adjuvant therapies.
中文翻译:

将 mRECIST 纳入 Metroticket 2.0 标准可提高对肝移植后肝细胞癌相关死亡的预测
背景 目的:对于大多数 LT 后预测模型而言,选择具有可接受复发风险的肝细胞癌 (HCC) 候选者进行肝移植 (LT) 的新辅助治疗反应的权重仍然部分未解决。本研究的目的是将放射学反应嵌入 Metroticket 2.0 模型中,用于“HCC 相关死亡”的 LT 后预测,以在现代临床场景中提供更多有用性。方法 纳入了 859 名接受新辅助治疗的移植患者 (2000-2015) 的数据。根据 mRECIST 标准审查 LT 前的最后一次放射学评估。应用了竞争风险分析。通过基于类别的净重分类改进 (NRI),探索了将放射反应纳入 Metroticket 2.0 的附加价值。结果 在 LT 之前的最后一次放射学评估中,41.3% 的患者诊断出完全缓解 (CR),24.9% 诊断出部分缓解/疾病稳定 (PR/SD),33.8% 诊断出疾病进展 (PD)。CR 患者的“HCC 相关死亡率”5 年率为 3.1%,PR/SD 患者为 9.6%,PD 患者为 13.4%(P<0.001)。Log10AFP(p<0.001)和肿瘤数目与直径之和/s(p<0.05)是PR/SD和PD患者“HCC相关死亡”的决定因素,具有不同的危害。为了保持 LT 后 5 年“HCC 相关死亡”的发生率 <30%,Metroticket 2.0 标准在某些 PR/SD 病例和所有 PD 病例中受到限制,正确重新分类了 9.4% 的死亡患者“HCC 相关死亡”,以 3.5% 的未发生事件的患者为代价。整体 NRI 为 5.8。结论 将 mRECIST 标准纳入 Metroticket 2.0 框架可以在判断接受新辅助治疗的 LT 候选人的资格时提供进一步的临床信息。
更新日期:2020-08-01
中文翻译:

将 mRECIST 纳入 Metroticket 2.0 标准可提高对肝移植后肝细胞癌相关死亡的预测
背景 目的:对于大多数 LT 后预测模型而言,选择具有可接受复发风险的肝细胞癌 (HCC) 候选者进行肝移植 (LT) 的新辅助治疗反应的权重仍然部分未解决。本研究的目的是将放射学反应嵌入 Metroticket 2.0 模型中,用于“HCC 相关死亡”的 LT 后预测,以在现代临床场景中提供更多有用性。方法 纳入了 859 名接受新辅助治疗的移植患者 (2000-2015) 的数据。根据 mRECIST 标准审查 LT 前的最后一次放射学评估。应用了竞争风险分析。通过基于类别的净重分类改进 (NRI),探索了将放射反应纳入 Metroticket 2.0 的附加价值。结果 在 LT 之前的最后一次放射学评估中,41.3% 的患者诊断出完全缓解 (CR),24.9% 诊断出部分缓解/疾病稳定 (PR/SD),33.8% 诊断出疾病进展 (PD)。CR 患者的“HCC 相关死亡率”5 年率为 3.1%,PR/SD 患者为 9.6%,PD 患者为 13.4%(P<0.001)。Log10AFP(p<0.001)和肿瘤数目与直径之和/s(p<0.05)是PR/SD和PD患者“HCC相关死亡”的决定因素,具有不同的危害。为了保持 LT 后 5 年“HCC 相关死亡”的发生率 <30%,Metroticket 2.0 标准在某些 PR/SD 病例和所有 PD 病例中受到限制,正确重新分类了 9.4% 的死亡患者“HCC 相关死亡”,以 3.5% 的未发生事件的患者为代价。整体 NRI 为 5.8。结论 将 mRECIST 标准纳入 Metroticket 2.0 框架可以在判断接受新辅助治疗的 LT 候选人的资格时提供进一步的临床信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号