Electrochimica Acta ( IF 6.6 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.electacta.2020.136080 Balamurugan Devadas , Jan Svoboda , Martin Krupička , Tomas Bystron
|
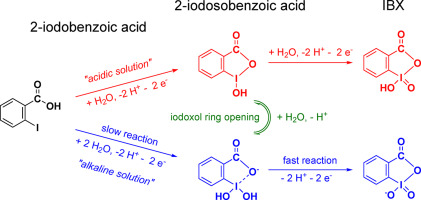
2-iodosobenzoic acid and especially IBX represent interesting selective and green hypervalent iodine oxidants. Mainly due to issues connected with their preparation, use of these compounds is limited to laboratory scale. From this point of view, the electrochemical synthesis represents interesting platform for inherently safer production of these highly valued oxidants. In order to provoke and allow more intense investigation in this direction, this work aimed to present basic aspects of electrochemical behaviour of these compounds in aqueus environment using several anode materials. A combination of various experimental approaches in combination with time-dependent density functional theory calculations allowed determining compounds speciation in a wide range of pH values. The most interesting in this sense is iodoxol ring opening after/during deprotonation of 2-iodosobenzoic acid to 2-iodosobenzoate. This process is accompanied by addition of water molecule to the iodine atom. Using voltammetry and controlled potential preparative batch electrolysis allowed constructing approximate form of Pourbaix diagram of the investigated compounds. Finally, diffusion coefficients of investigated compounds and anodic charge transfer coefficients of oxidation reactions were determined based on detailed analysis of linear voltammograms. The results provide a solid base for further investigation of electrochemical synthesis of 2-iodosobenzoic acid and IBX in aqueous environment.
中文翻译:

2-碘苯甲酸和2-碘苯甲酸阳极氧化在水环境中的电化学和光谱研究
2-碘代苯甲酸,尤其是IBX代表了有趣的选择性和绿色高价碘氧化剂。主要由于与制备有关的问题,这些化合物的使用仅限于实验室规模。从这个角度来看,电化学合成代表了有趣的平台,这些平台本质上更安全地生产了这些高价值的氧化剂。为了激发并允许对此方向进行更深入的研究,这项工作旨在介绍使用几种阳极材料在水性环境中这些化合物的电化学行为的基本方面。各种实验方法的结合以及与时间相关的密度泛函理论计算相结合,可以在宽范围的pH值中确定化合物的形态。在这种意义上,最有趣的是在2-碘代苯甲酸去质子化为2-碘代苯甲酸酯后/期间的碘醇开环。该过程伴随有将水分子添加到碘原子上。使用伏安法和控制电位的分批制备电解可以构建所研究化合物的Pourbaix图的近似形式。最后,基于线性伏安图的详细分析,确定了所研究化合物的扩散系数和氧化反应的阳极电荷转移系数。该结果为进一步研究2-碘代苯甲酸和IBX在水环境中的电化学合成提供了坚实的基础。使用伏安法和控制电位的分批制备电解可以构建所研究化合物的Pourbaix图的近似形式。最后,基于线性伏安图的详细分析,确定了所研究化合物的扩散系数和氧化反应的阳极电荷转移系数。该结果为进一步研究2-碘代苯甲酸和IBX在水环境中的电化学合成提供了坚实的基础。使用伏安法和控制电位的分批制备电解可以构建所研究化合物的Pourbaix图的近似形式。最后,基于线性伏安图的详细分析,确定了所研究化合物的扩散系数和氧化反应的阳极电荷转移系数。该结果为进一步研究2-碘代苯甲酸和IBX在水环境中的电化学合成提供了坚实的基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号