Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.tetlet.2020.151853 Mitsuru Kitamura , Tomoaki Nishimura , Kota Otsuka , Hirokazu Shimooka , Tatsuo Okauchi
|
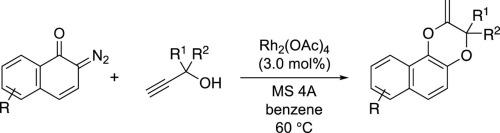
A Rh(II)-catalyzed formal [3+3] cyclization of diazonaphthoquinones and propargyl alcohol is reported to afford 2,3-dihydro-1,4-benzodioxins. Various terminal propargyl alcohols react with diazonapthoquinone in the presence of Rh2(OAc)4 to give the corresponding dihydrodioxins in good to high yields. However, dihydrodioxins are not formed in the reaction of internal propargyl alcohols, and the O–H insertion product and 2,5-dihydrofurans are formed as the main product(s) depending on the terminal substituent. 2,3-Dihydro-1,4-benzodioxins are proposed to be formed via Rh(II)-catalyzed intermolecular oxonium ylide formation and subsequent 6-exo-dig cyclization with the internal alkynyl group.
中文翻译:

Rh(II)催化的重氮萘醌和炔丙醇的正式[3 + 3]环加成反应:2,3-二氢萘-1,4-二恶英衍生物的合成
据报道,Rh(II)催化的重氮萘醌和炔丙醇的正式[3 + 3]环化反应可提供2,3-二氢-1,4-苯并二恶英。各种末端炔丙醇在Rh 2(OAc)4的存在下与重氮萘醌反应,以高至高收率得到相应的二氢二恶英。但是,在内部炔丙醇的反应中不会形成二氢二恶英,取决于末端取代基,OH插入产物和2,5-二氢呋喃是主要产物。提出通过Rh(II)催化的分子间氧鎓叶立德形成和随后的内部炔基6- exo - dig环化反应形成2,3-二氢-1,4-苯并二恶英。











































 京公网安备 11010802027423号
京公网安备 11010802027423号