当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nucleolin Discriminates Drastically between Long-Loop and Short-Loop Quadruplexes.
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-19 , DOI: 10.1021/acs.biochem.9b01094 Abhijit Saha 1, 2 , Patricia Duchambon 1, 2 , Vanessa Masson 3 , Damarys Loew 3 , Sophie Bombard 1, 2 , Marie-Paule Teulade-Fichou 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-19 , DOI: 10.1021/acs.biochem.9b01094 Abhijit Saha 1, 2 , Patricia Duchambon 1, 2 , Vanessa Masson 3 , Damarys Loew 3 , Sophie Bombard 1, 2 , Marie-Paule Teulade-Fichou 1, 2
Affiliation
|
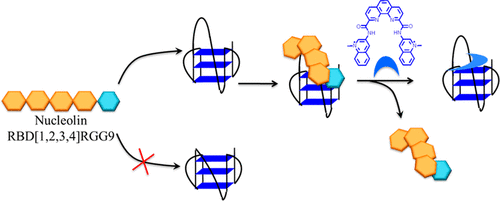
We investigate herein the interaction between nucleolin (NCL) and a set of G4 sequences derived from the CEB25 human minisatellite that adopt a parallel topology while differing in the length of the central loop (from nine nucleotides to one nucleotide). It is revealed that NCL strongly binds to long-loop (five to nine nucleotides) G4 while interacting weakly with the shorter variants (loop with fewer than three nucleotides). Photo-cross-linking experiments using 5-bromo-2′-deoxyuridine (BrU)-modified sequences further confirmed the loop-length dependency, thereby indicating that the WT-CEB25-L191 (nine-nucleotide loop) is the best G4 substrate. Quantitative proteomic analysis (LC-MS/MS) of the product(s) obtained by photo-cross-linking NCL to this sequence enabled the identification of one contact site corresponding to a 15-amino acid fragment located in helix α2 of RNA binding domain 2 (RBD2), which sheds light on the role of this structural element in G4-loop recognition. Then, the ability of a panel of benchmark G4 ligands to prevent the NCL–G4 interaction was explored. It was found that only the most potent ligand PhenDC3 can inhibit NCL binding, thereby suggesting that the terminal guanine quartet is also a strong determinant of G4 recognition, putatively through interaction with the RGG domain. This study describes the molecular mechanism by which NCL recognizes G4-containing long loops and leads to the proposal of a model implying a concerted action of RBD2 and RGG domains to achieve specific G4 recognition via a dual loop–quartet interaction.
中文翻译:

核仁素对长环和短环四链体有很大的区别。
我们在此研究核仁素 (NCL) 和一组源自 CEB25 人类小卫星的 G4 序列之间的相互作用,这些序列采用平行拓扑,但中心环的长度不同(从 9 个核苷酸到 1 个核苷酸)。研究表明,NCL 与长环(5 至 9 个核苷酸)G4 强烈结合,而与较短的变体(少于 3 个核苷酸的环)相互作用较弱。使用 5-溴-2'-脱氧尿苷 (BrU) 修饰序列的光交联实验进一步证实了环长度依赖性,从而表明 WT-CEB25-L191(九核苷酸环)是最好的 G4 底物。对通过光交联 NCL 与该序列获得的产物进行定量蛋白质组分析 (LC-MS/MS),能够鉴定出与位于 RNA 结合结构域螺旋 α2 中的 15 个氨基酸片段相对应的一个接触位点2 (RBD2),揭示了该结构元件在 G4 环识别中的作用。然后,探索了一组基准 G4 配体阻止 NCL-G4 相互作用的能力。结果发现,只有最有效的配体 PhenDC3 才能抑制 NCL 结合,从而表明末端鸟嘌呤四联体也是 G4 识别的强大决定因素,推测是通过与 RGG 结构域相互作用。这项研究描述了 NCL 识别含有 G4 的长环的分子机制,并提出了一个模型,该模型暗示 RBD2 和 RGG 结构域的协同作用,通过双环-四联体相互作用实现特定的 G4 识别。
更新日期:2020-03-20
中文翻译:

核仁素对长环和短环四链体有很大的区别。
我们在此研究核仁素 (NCL) 和一组源自 CEB25 人类小卫星的 G4 序列之间的相互作用,这些序列采用平行拓扑,但中心环的长度不同(从 9 个核苷酸到 1 个核苷酸)。研究表明,NCL 与长环(5 至 9 个核苷酸)G4 强烈结合,而与较短的变体(少于 3 个核苷酸的环)相互作用较弱。使用 5-溴-2'-脱氧尿苷 (BrU) 修饰序列的光交联实验进一步证实了环长度依赖性,从而表明 WT-CEB25-L191(九核苷酸环)是最好的 G4 底物。对通过光交联 NCL 与该序列获得的产物进行定量蛋白质组分析 (LC-MS/MS),能够鉴定出与位于 RNA 结合结构域螺旋 α2 中的 15 个氨基酸片段相对应的一个接触位点2 (RBD2),揭示了该结构元件在 G4 环识别中的作用。然后,探索了一组基准 G4 配体阻止 NCL-G4 相互作用的能力。结果发现,只有最有效的配体 PhenDC3 才能抑制 NCL 结合,从而表明末端鸟嘌呤四联体也是 G4 识别的强大决定因素,推测是通过与 RGG 结构域相互作用。这项研究描述了 NCL 识别含有 G4 的长环的分子机制,并提出了一个模型,该模型暗示 RBD2 和 RGG 结构域的协同作用,通过双环-四联体相互作用实现特定的 G4 识别。









































 京公网安备 11010802027423号
京公网安备 11010802027423号