当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure-Property Relationships in Unsymmetric Bis(antiaromatics): Who Wins the Battle between Pentalene and Benzocyclobutadiene?†.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-19 , DOI: 10.1021/acs.joc.9b03119 Péter J Mayer 1, 2 , Ouissam El Bakouri 3 , Tamás Holczbauer 4 , Gergely F Samu 5 , Csaba Janáky 5 , Henrik Ottosson 3 , Gábor London 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-19 , DOI: 10.1021/acs.joc.9b03119 Péter J Mayer 1, 2 , Ouissam El Bakouri 3 , Tamás Holczbauer 4 , Gergely F Samu 5 , Csaba Janáky 5 , Henrik Ottosson 3 , Gábor London 1
Affiliation
|
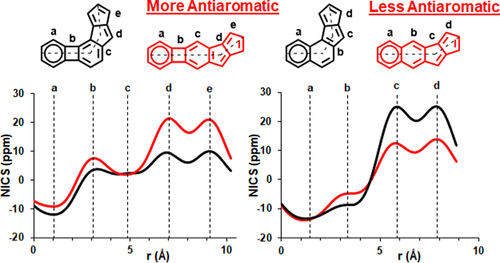
According to the currently accepted structure–property relationships, aceno-pentalenes with an angular shape (fused to the 1,2-bond of the acene) exhibit higher antiaromaticity than those with a linear shape (fused to the 2,3-bond of the acene). To explore and expand the current view, we designed and synthesized molecules where two isomeric, yet, different, 8π antiaromatic subunits, a benzocyclobutadiene (BCB) and a pentalene, are combined into, respectively, an angular and a linear topology via an unsaturated six-membered ring. The antiaromatic character of the molecules is supported experimentally by 1H NMR, UV–vis, and cyclic voltammetry measurements and X-ray crystallography. The experimental results are further confirmed by theoretical studies including the calculation of several aromaticity indices (NICS, ACID, HOMA, FLU, MCI). In the case of the angular molecule, double bond-localization within the connecting six-membered ring resulted in reduced antiaromaticity of both the BCB and pentalene subunits, while the linear structure provided a competitive situation for the two unequal [4n]π subunits. We found that in the latter case the BCB unit alleviated its unfavorable antiaromaticity more efficiently, leaving the pentalene with strong antiaromaticity. Thus, a reversed structure–antiaromaticity relationship when compared to aceno-pentalenes was achieved.
中文翻译:

非对称双(抗芳香族化合物)中的结构-性质关系:谁赢得了戊烯和苯并环丁二烯之间的战斗?†。
根据目前公认的结构-性质关系,具有角形(与并苯的1,2-键键合)的乙酰戊戊烯比具有线性(与2,3-键的苯并酮键合)具有更高的抗芳香性。粉刺)。为了探索和扩展当前的观点,我们设计并合成了分子,其中两个同分异构但不同的8π抗芳香亚基苯并环丁二烯(BCB)和戊烯分别通过不饱和六价角和线性拓扑结合环。分子的抗芳香特性在实验上由1支持H NMR,UV-vis和循环伏安法测量以及X射线晶体学。理论研究进一步证实了该实验结果,包括对几种芳香指数(NICS,ACID,HOMA,FLU,MCI)的计算。在有角分子的情况下,连接的六元环内的双键局部化导致BCB和戊烯亚基的抗芳香性降低,而线性结构为两个不相等的[4 n ]π亚基提供了竞争态势。我们发现,在后一种情况下,BCB单元可以更有效地缓解其不利的抗芳香性,使戊烯具有强大的抗芳香性。因此,与乙酰戊戊烯相比,获得了反向的结构-抗芳香性关系。
更新日期:2020-04-24
中文翻译:

非对称双(抗芳香族化合物)中的结构-性质关系:谁赢得了戊烯和苯并环丁二烯之间的战斗?†。
根据目前公认的结构-性质关系,具有角形(与并苯的1,2-键键合)的乙酰戊戊烯比具有线性(与2,3-键的苯并酮键合)具有更高的抗芳香性。粉刺)。为了探索和扩展当前的观点,我们设计并合成了分子,其中两个同分异构但不同的8π抗芳香亚基苯并环丁二烯(BCB)和戊烯分别通过不饱和六价角和线性拓扑结合环。分子的抗芳香特性在实验上由1支持H NMR,UV-vis和循环伏安法测量以及X射线晶体学。理论研究进一步证实了该实验结果,包括对几种芳香指数(NICS,ACID,HOMA,FLU,MCI)的计算。在有角分子的情况下,连接的六元环内的双键局部化导致BCB和戊烯亚基的抗芳香性降低,而线性结构为两个不相等的[4 n ]π亚基提供了竞争态势。我们发现,在后一种情况下,BCB单元可以更有效地缓解其不利的抗芳香性,使戊烯具有强大的抗芳香性。因此,与乙酰戊戊烯相比,获得了反向的结构-抗芳香性关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号