当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Control of Gene-Engineered T cell Activity Through Covalent Attachment of Targeting Ligands to a Universal Immune Receptor
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-19 , DOI: 10.1021/jacs.9b11622 Nicholas G. Minutolo , Prannda Sharma , Mathilde Poussin , Lauren C. Shaw , Daniel P. Brown , Erin E. Hollander , Anže Smole , Alba Rodriguez-Garcia , James Z. Hui , Fabiana Zappala , Andrew Tsourkas , Daniel J. Powell
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-19 , DOI: 10.1021/jacs.9b11622 Nicholas G. Minutolo , Prannda Sharma , Mathilde Poussin , Lauren C. Shaw , Daniel P. Brown , Erin E. Hollander , Anže Smole , Alba Rodriguez-Garcia , James Z. Hui , Fabiana Zappala , Andrew Tsourkas , Daniel J. Powell
|
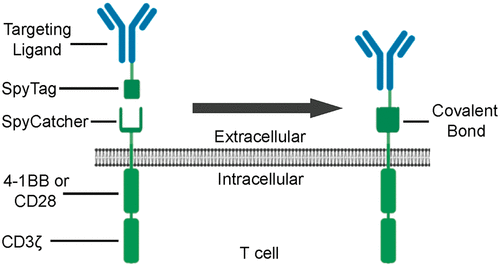
Universal immune receptors represent a rapidly emerging form of adoptive T cell therapy with the potential to overcome safety and antigen escape challenges faced by conventional chimeric antigen receptor (CAR) T cell therapy. By decoupling antigen recognition and T cell signaling domains via bifunctional antigen-specific targeting ligands, universal immune receptors can regulate T cell effector function and target multiple antigens with a single receptor. Here, we describe the development of the SpyCatcher immune receptor, the first universal immune receptor that allows for post-translational covalent attachment of targeting ligands at the T cell surface through the application of SpyCatcher-SpyTag chemistry. The SpyCatcher immune receptor redirected primary human T cells against a variety of tumor antigens via the addition of SpyTag-labeled targeting ligands, both in vitro and in vivo. SpyCatcher T cell activity relied upon the presence of both target antigen and SpyTag-labeled targeting ligand, allowing for dose-dependent control of function. Mutational disruption of covalent bond formation between the receptor and the targeting ligand still permitted redirected T cell function but significantly compromised antitumor function. Thus, the SpyCatcher immune receptor allows for rapid antigen-specific receptor assembly, multi-antigen targeting, and controllable T cell activity.
中文翻译:

通过靶向配体与通用免疫受体的共价连接对基因工程 T 细胞活性进行定量控制
通用免疫受体代表了一种快速出现的过继性 T 细胞疗法,具有克服传统嵌合抗原受体 (CAR) T 细胞疗法面临的安全性和抗原逃逸挑战的潜力。通过双功能抗原特异性靶向配体将抗原识别和 T 细胞信号结构域解偶联,通用免疫受体可以调节 T 细胞效应器功能并用单个受体靶向多种抗原。在这里,我们描述了 SpyCatcher 免疫受体的发展,这是第一个允许通过应用 SpyCatcher-SpyTag 化学在 T 细胞表面翻译后共价连接靶向配体的通用免疫受体。SpyCatcher 免疫受体通过添加 SpyTag 标记的靶向配体,在体外和体内重定向原代人类 T 细胞对抗各种肿瘤抗原。SpyCatcher T 细胞活性依赖于靶抗原和 SpyTag 标记的靶向配体的存在,允许剂量依赖性控制功能。受体和靶向配体之间共价键形成的突变破坏仍然允许重定向 T 细胞功能,但显着损害抗肿瘤功能。因此,SpyCatcher 免疫受体允许快速抗原特异性受体组装、多抗原靶向和可控的 T 细胞活性。允许对功能进行剂量依赖性控制。受体和靶向配体之间共价键形成的突变破坏仍然允许重定向 T 细胞功能,但显着损害抗肿瘤功能。因此,SpyCatcher 免疫受体允许快速抗原特异性受体组装、多抗原靶向和可控的 T 细胞活性。允许对功能进行剂量依赖性控制。受体和靶向配体之间共价键形成的突变破坏仍然允许重定向 T 细胞功能,但显着损害抗肿瘤功能。因此,SpyCatcher 免疫受体允许快速抗原特异性受体组装、多抗原靶向和可控的 T 细胞活性。
更新日期:2020-03-19
中文翻译:

通过靶向配体与通用免疫受体的共价连接对基因工程 T 细胞活性进行定量控制
通用免疫受体代表了一种快速出现的过继性 T 细胞疗法,具有克服传统嵌合抗原受体 (CAR) T 细胞疗法面临的安全性和抗原逃逸挑战的潜力。通过双功能抗原特异性靶向配体将抗原识别和 T 细胞信号结构域解偶联,通用免疫受体可以调节 T 细胞效应器功能并用单个受体靶向多种抗原。在这里,我们描述了 SpyCatcher 免疫受体的发展,这是第一个允许通过应用 SpyCatcher-SpyTag 化学在 T 细胞表面翻译后共价连接靶向配体的通用免疫受体。SpyCatcher 免疫受体通过添加 SpyTag 标记的靶向配体,在体外和体内重定向原代人类 T 细胞对抗各种肿瘤抗原。SpyCatcher T 细胞活性依赖于靶抗原和 SpyTag 标记的靶向配体的存在,允许剂量依赖性控制功能。受体和靶向配体之间共价键形成的突变破坏仍然允许重定向 T 细胞功能,但显着损害抗肿瘤功能。因此,SpyCatcher 免疫受体允许快速抗原特异性受体组装、多抗原靶向和可控的 T 细胞活性。允许对功能进行剂量依赖性控制。受体和靶向配体之间共价键形成的突变破坏仍然允许重定向 T 细胞功能,但显着损害抗肿瘤功能。因此,SpyCatcher 免疫受体允许快速抗原特异性受体组装、多抗原靶向和可控的 T 细胞活性。允许对功能进行剂量依赖性控制。受体和靶向配体之间共价键形成的突变破坏仍然允许重定向 T 细胞功能,但显着损害抗肿瘤功能。因此,SpyCatcher 免疫受体允许快速抗原特异性受体组装、多抗原靶向和可控的 T 细胞活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号