当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of plasma concentrations of paracetamol, metacetamol, and orthocetamol in Japanese racehorses using liquid chromatography-electrospray ionisation-tandem mass spectrometry.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-04-01 , DOI: 10.1002/dta.2792 Hideaki Ishii 1, 2 , Taku Obara 2 , Isao Kijima-Suda 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-04-01 , DOI: 10.1002/dta.2792 Hideaki Ishii 1, 2 , Taku Obara 2 , Isao Kijima-Suda 1
Affiliation
|
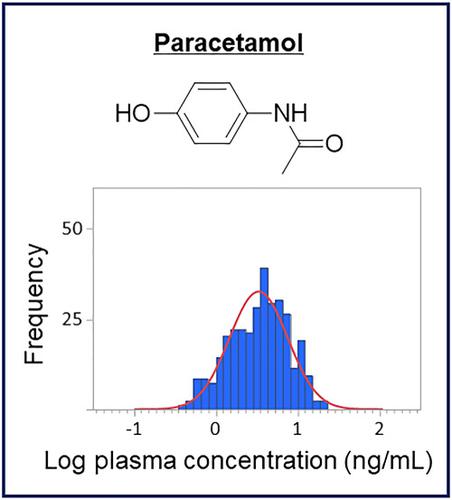
Paracetamol is used widely as an over‐the‐counter analgesic and antipyretic medication for humans, but not for Japanese racehorses. Paracetamol can be an environmental substance, and is found together with its two isomers, metacetamol and orthocetamol, in equine urine. However, the sources and routes of paracetamol exposure remain unclear. To control the misuse of paracetamol, it is appropriate to establish residue limits for paracetamol to differentiate the administration of paracetamol from its environmental levels. In this study, we developed and validated a quantitative method for paracetamol, metacetamol, and orthocetamol in equine plasma using liquid chromatography–electrospray ionization–tandem mass spectrometry and applied it to postrace samples from 320 Japanese racehorses for approximately 1 year. In addition, we conducted feed analysis and related pharmacokinetics simulations to evaluate the contributions from exposure via feed. The hydrolyzed plasma concentrations of paracetamol, metacetamol, and orthocetamol ranged from 0.787 to 39.8 ng/mL (median 5.87 ng/mL), 0 to 2.13 ng/mL (0.347 ng/mL), and 1.98 to 82.8 ng/mL (16.6 ng/mL), respectively. Such low concentrations of paracetamol were deemed irrelevant to therapeutic effect. Based on statistical analysis, the preliminary Japanese residue limits of unhydrolyzed and hydrolyzed paracetamol were determined to be 70.5 ng/mL and 112 ng/mL, respectively, in plasma, at a risk factor of 1 in 10,000. Furthermore, we detected paracetamol and orthocetamol in feed samples. A pharmacokinetics simulation showed that the presence of orthocetamol is most probably related to daily feed rations. As for paracetamol, feed can be one of the sources and other possible sources require further investigation.
中文翻译:

使用液相色谱-电喷雾电离-串联质谱法研究日本赛马中扑热息痛,扑热息痛和邻苯二酚的血浆浓度。
扑热息痛广泛用于人类的非处方镇痛和解热药物,但不适用于日本赛马。扑热息痛可以是一种环境物质,可以在马尿中与扑热息痛和它的两种异构体,扑热息痛和邻乙酰氨基酚一起发现。但是,扑热息痛暴露的来源和途径仍不清楚。为了控制对乙酰氨基酚的滥用,有必要确定对乙酰氨基酚的残留限量,以区分对乙酰氨基酚的施用与其环境水平。在这项研究中,我们开发并验证了使用液相色谱-电喷雾电离-串联质谱法测定马血浆中扑热息痛,扑热息痛和邻苯二酚的定量方法,并将其应用于约320种日本赛马的赛后样品。此外,我们进行了饲料分析和相关的药代动力学模拟,以评估通过饲料暴露造成的影响。扑热息痛,扑热息痛和邻苯二酚的水解血浆浓度范围为0.787至39.8 ng / mL(中值5.87 ng / mL),0至2.13 ng / mL(0.347 ng / mL)和1.98至82.8 ng / mL(16.6 ng) / mL)。如此低的对乙酰氨基酚浓度被认为与治疗效果无关。根据统计分析,血浆中未水解和水解对乙酰氨基酚的日本残留限量初步确定为分别为70.5 ng / mL和112 ng / mL,风险因子为10,000分之一。此外,我们在饲料样品中检测到了扑热息痛和邻苯二酚。药代动力学模拟表明邻苯二酚的存在最有可能与日饲料配比有关。至于扑热息痛,
更新日期:2020-04-01
中文翻译:

使用液相色谱-电喷雾电离-串联质谱法研究日本赛马中扑热息痛,扑热息痛和邻苯二酚的血浆浓度。
扑热息痛广泛用于人类的非处方镇痛和解热药物,但不适用于日本赛马。扑热息痛可以是一种环境物质,可以在马尿中与扑热息痛和它的两种异构体,扑热息痛和邻乙酰氨基酚一起发现。但是,扑热息痛暴露的来源和途径仍不清楚。为了控制对乙酰氨基酚的滥用,有必要确定对乙酰氨基酚的残留限量,以区分对乙酰氨基酚的施用与其环境水平。在这项研究中,我们开发并验证了使用液相色谱-电喷雾电离-串联质谱法测定马血浆中扑热息痛,扑热息痛和邻苯二酚的定量方法,并将其应用于约320种日本赛马的赛后样品。此外,我们进行了饲料分析和相关的药代动力学模拟,以评估通过饲料暴露造成的影响。扑热息痛,扑热息痛和邻苯二酚的水解血浆浓度范围为0.787至39.8 ng / mL(中值5.87 ng / mL),0至2.13 ng / mL(0.347 ng / mL)和1.98至82.8 ng / mL(16.6 ng) / mL)。如此低的对乙酰氨基酚浓度被认为与治疗效果无关。根据统计分析,血浆中未水解和水解对乙酰氨基酚的日本残留限量初步确定为分别为70.5 ng / mL和112 ng / mL,风险因子为10,000分之一。此外,我们在饲料样品中检测到了扑热息痛和邻苯二酚。药代动力学模拟表明邻苯二酚的存在最有可能与日饲料配比有关。至于扑热息痛,











































 京公网安备 11010802027423号
京公网安备 11010802027423号