当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Palladium‐Catalyzed Tandem Reaction of Cyanomethyl Benzoates with Arylboronic Acids: Synthesis of Oxazoles and Isocoumarins
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-03 , DOI: 10.1002/adsc.202000125 Ling Dai 1 , Shuling Yu 1 , Wenzhang Xiong 1 , Zhongyan Chen 1 , Tong Xu 1 , Yinlin Shao 1 , Jiuxi Chen 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-03 , DOI: 10.1002/adsc.202000125 Ling Dai 1 , Shuling Yu 1 , Wenzhang Xiong 1 , Zhongyan Chen 1 , Tong Xu 1 , Yinlin Shao 1 , Jiuxi Chen 1
Affiliation
|
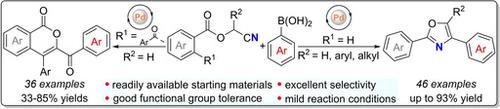
A palladium‐catalyzed tandem reaction of cyanomethyl benzoates with arylboronic acids has been achieved. Substitution at the 2‐position of cyanomethyl benzoates was found to be crucial for the selective synthesis of oxazoles and isocoumarins. Cyanomethyl benzoates afforded 2,4‐diaryloxazoles as products, while 2‐benzoyl‐substituted cyanomethyl benzoates delivered 3‐benzoyl‐4‐aryl‐isocoumarins selectively. Furthermore, a possible mechanism for the selective reaction of cyanomethyl benzoates with arylboronic acids was discussed.
中文翻译:

氰基苯甲酸酯与芳基硼酸的不同钯催化串联反应:恶唑和异香豆素的合成
已实现了氰基甲基苯甲酸酯与芳基硼酸的钯催化串联反应。已发现在氰基甲基苯甲酸酯的2位取代对于选择合成恶唑和异香豆素至关重要。氰基苯甲酸甲酯提供了2,4-二芳基恶唑,而2-苯甲酰基取代的氰基甲基苯甲酸酯则选择性地提供了3-苯甲酰基-4-芳基-异香豆素。此外,讨论了氰基甲基苯甲酸酯与芳基硼酸选择性反应的可能机理。
更新日期:2020-04-03
中文翻译:

氰基苯甲酸酯与芳基硼酸的不同钯催化串联反应:恶唑和异香豆素的合成
已实现了氰基甲基苯甲酸酯与芳基硼酸的钯催化串联反应。已发现在氰基甲基苯甲酸酯的2位取代对于选择合成恶唑和异香豆素至关重要。氰基苯甲酸甲酯提供了2,4-二芳基恶唑,而2-苯甲酰基取代的氰基甲基苯甲酸酯则选择性地提供了3-苯甲酰基-4-芳基-异香豆素。此外,讨论了氰基甲基苯甲酸酯与芳基硼酸选择性反应的可能机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号