当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Enantioselective Synthesis of Chiral Carbocyclic Nucleosides via Palladium‐Catalyzed Asymmetric Allylic Amination of Alicyclic MBH Adducts with Purines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-18 , DOI: 10.1002/adsc.202000088 Bo Kang 1 , Qi‐Ying Zhang 1 , Gui‐Rong Qu 1 , Hai‐Ming Guo 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-18 , DOI: 10.1002/adsc.202000088 Bo Kang 1 , Qi‐Ying Zhang 1 , Gui‐Rong Qu 1 , Hai‐Ming Guo 1
Affiliation
|
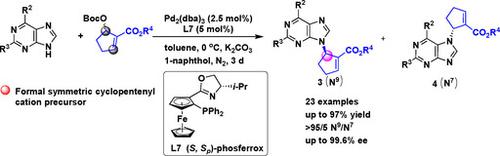
The enantioselective synthesis of carbocyclic nucleosides through the palladium‐catalyzed asymmetric allylic amination of alicyclic Morita‐Baylis‐Hillman (MBH) adducts with purines was successfully developed. With a combination of Pd2(dba)3/L7 as catalyst, various optically active carbocyclic nucleosides featuring a C=C double bond in the carbocycle moiety were obtained in high yields (up to 97%) with excellent N9/N7‐selectivities (>95/5) and enantioselectivities (up to >99.6%). In addition, these nucleoside analogs allowed for rapid transformation to a variety of other interesting structurally diverse chiral carbocyclic nucleosides.
中文翻译:

通过钯催化嘌呤与脂环族MBH加合物的不对称烯丙基胺的手性碳环核苷的对映选择性合成
通过钯催化的嘌呤与脂环式森田-贝利斯-希尔曼(MBH)加合物的钯催化不对称烯丙基胺化,成功开发了碳环核苷的对映选择性合成。结合使用Pd 2(dba)3 / L7作为催化剂,可以以高收率(高达97%)和优异的N 9 / N 7-获得各种在碳环部分具有C = C双键的旋光碳环核苷。选择性(> 95/5)和对映选择性(> 99.6%)。另外,这些核苷类似物允许快速转化为多种其他有趣的结构上不同的手性碳环核苷。
更新日期:2020-03-18
中文翻译:

通过钯催化嘌呤与脂环族MBH加合物的不对称烯丙基胺的手性碳环核苷的对映选择性合成
通过钯催化的嘌呤与脂环式森田-贝利斯-希尔曼(MBH)加合物的钯催化不对称烯丙基胺化,成功开发了碳环核苷的对映选择性合成。结合使用Pd 2(dba)3 / L7作为催化剂,可以以高收率(高达97%)和优异的N 9 / N 7-获得各种在碳环部分具有C = C双键的旋光碳环核苷。选择性(> 95/5)和对映选择性(> 99.6%)。另外,这些核苷类似物允许快速转化为多种其他有趣的结构上不同的手性碳环核苷。










































 京公网安备 11010802027423号
京公网安备 11010802027423号