当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atmospheric chemistry of thiourea: nucleation with urea and roles in NO2 hydrolysis
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020/03/19 , DOI: 10.1039/c9cp04300d Shuang Ni 1, 2, 3, 4, 5 , Feng-Yang Bai 6, 7, 8, 9, 10 , Xiu-Mei Pan 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020/03/19 , DOI: 10.1039/c9cp04300d Shuang Ni 1, 2, 3, 4, 5 , Feng-Yang Bai 6, 7, 8, 9, 10 , Xiu-Mei Pan 1, 2, 3, 4, 5
Affiliation
|
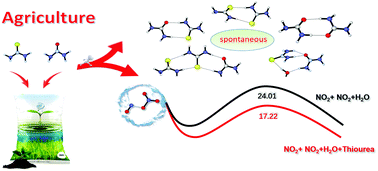
Nitrogenous particle participation in the formation of clusters has attracted considerable attention from numerous researchers in recent years. Urea and thiourea (TU), as the common fertilizers in agriculture, have a significant impact on the atmospheric environment, whereas their implications have not been comprehended widely. Herein, we have used quantum calculations and ABCluster to explore the potential roles of thiourea and urea in particle formation events. A vital implication of these results is that they may contribute toward particle formation in marine environments and Asia region where the concentration of thiourea and urea has been increasing for a few years. Furthermore, the mechanisms of NO2 hydrolysis in the presence of thiourea and subsequent reactions were studied deeply. The results indicate that, although these reactions are not thermodynamically favorable at 298.15 K under homogeneous gas-phase conditions, thiourea may promote the hydrolysis of NO2 in heterogeneous environments containing very high concentrations of these molecules. The kinetics analysis shows that the rate constants of the hydrolysis reaction catalyzed by thiourea with N2O4–W and TU–W are about 2–5 and 1–2 orders of magnitude faster than those of the naked reaction. Thiourea nitrate and its aquo-complex were also studied, and the results suggest that the reaction produced an acid–base complex in which the trans- configuration is the final form for nitrous acid. We hope that these findings would inspire field measurements for detecting urea and thiourea in the troposphere.
中文翻译:

硫脲的大气化学:尿素成核及其在NO2水解中的作用
近年来,氮粒子参与团簇的形成引起了众多研究者的极大关注。尿素和硫脲(TU)作为农业上的常见肥料,对大气环境具有重大影响,但是其影响尚未得到广泛的理解。在本文中,我们使用了量子计算和ABCluster来探索硫脲和尿素在颗粒形成事件中的潜在作用。这些结果的关键意义在于,它们可能会导致海洋环境和亚洲地区中硫脲和尿素的浓度一直在增加的颗粒形成。此外,NO 2的机理对硫脲存在下的水解和后续反应进行了深入研究。结果表明,尽管这些反应在均相气相条件下在298.15 K时在热力学上不利,但硫脲可能在含有非常高浓度这些分子的非均质环境中促进NO 2水解。动力学分析表明,硫脲与N 2 O 4 –W和TU–W催化的水解反应速率常数比裸反应快约2–5和1-2个数量级。还研究了硝酸硫脲及其水族络合物,结果表明该反应产生了酸碱络合物,其中反式-配置是亚硝酸的最终形式。我们希望这些发现将启发对流层中现场检测尿素和硫脲。
更新日期:2020-04-15
中文翻译:

硫脲的大气化学:尿素成核及其在NO2水解中的作用
近年来,氮粒子参与团簇的形成引起了众多研究者的极大关注。尿素和硫脲(TU)作为农业上的常见肥料,对大气环境具有重大影响,但是其影响尚未得到广泛的理解。在本文中,我们使用了量子计算和ABCluster来探索硫脲和尿素在颗粒形成事件中的潜在作用。这些结果的关键意义在于,它们可能会导致海洋环境和亚洲地区中硫脲和尿素的浓度一直在增加的颗粒形成。此外,NO 2的机理对硫脲存在下的水解和后续反应进行了深入研究。结果表明,尽管这些反应在均相气相条件下在298.15 K时在热力学上不利,但硫脲可能在含有非常高浓度这些分子的非均质环境中促进NO 2水解。动力学分析表明,硫脲与N 2 O 4 –W和TU–W催化的水解反应速率常数比裸反应快约2–5和1-2个数量级。还研究了硝酸硫脲及其水族络合物,结果表明该反应产生了酸碱络合物,其中反式-配置是亚硝酸的最终形式。我们希望这些发现将启发对流层中现场检测尿素和硫脲。



























 京公网安备 11010802027423号
京公网安备 11010802027423号