PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-03-18 , DOI: 10.1371/journal.ppat.1008397 Xin Wu 1 , Amelia R I Lindsey 2, 3 , Paramita Chatterjee 1 , John H Werren 4 , Richard Stouthamer 2 , Soojin V Yi 1
|
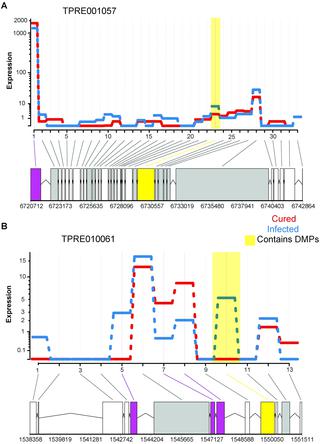
Wolbachia are maternally transmitted intracellular bacteria that induce a range of pathogenic and fitness-altering effects on insect and nematode hosts. In parasitoid wasps of the genus Trichogramma, Wolbachia infection induces asexual production of females, thus increasing transmission of Wolbachia. It has been hypothesized that Wolbachia infection accompanies a modification of the host epigenome. However, to date, data on genome-wide epigenomic changes associated with Wolbachia are limited, and are often confounded by background genetic differences. Here, we took sexually reproducing Trichogramma free of Wolbachia and introgressed their genome into a Wolbachia-infected cytoplasm, converting them to Wolbachia-mediated asexuality. Wolbachia was then cured from replicates of these introgressed lines, allowing us to examine the genome-wide effects of wasps newly converted to asexual reproduction while controlling for genetic background. We thus identified gene expression and DNA methylation changes associated with Wolbachia-infection. We found no overlaps between differentially expressed genes and differentially methylated genes, indicating that Wolbachia-infection associated DNA methylation change does not directly modulate levels of gene expression. Furthermore, genes affected by these mechanisms exhibit distinct evolutionary histories. Genes differentially methylated due to the infection tended to be evolutionarily conserved. In contrast, differentially expressed genes were significantly more likely to be unique to the Trichogramma lineage, suggesting host-specific transcriptomic responses to infection. Nevertheless, we identified several novel aspects of Wolbachia-associated DNA methylation changes. Differentially methylated genes included those involved in oocyte development and chromosome segregation. Interestingly, Wolbachia-infection was associated with higher levels of DNA methylation. Additionally, Wolbachia infection reduced overall variability in gene expression, even after accounting for the effect of DNA methylation. We also identified specific cases where alternative exon usage was associated with DNA methylation changes with Wolbachia infection. These results begin to reveal distinct genes and molecular pathways subject to Wolbachia induced epigenetic modification and/or host responses to Wolbachia-infection.
中文翻译:

与沃尔巴克氏体介导的无性相关的明显的表观基因组学和转录组学修饰。
Wolbachia是母源传播的细胞内细菌,可对昆虫和线虫宿主产生一系列致病和改变健康的作用。在赤眼蜂属的寄生性黄蜂中,Wolbachia感染诱导雌性无性繁殖,因此增加了Wolbachia的传播。已经假设沃尔巴氏菌感染伴随宿主表观基因组的修饰。然而,迄今为止,与沃尔巴克氏菌有关的全基因组表观基因组变化的数据是有限的,并且常常被背景遗传差异所混淆。在这里,我们采集了不含Wolbachia的有性生殖赤眼蜂然后将其基因组渗入感染了Wolbachia的细胞质中,然后将其转化为Wolbachia介导的无性恋。然后从这些渗入系的复制品中治愈了沃尔巴克氏菌,使我们能够在控制遗传背景的同时检查新近转化为无性繁殖的黄蜂的全基因组效应。因此,我们确定了与沃尔巴克氏菌感染相关的基因表达和DNA甲基化变化。我们发现差异表达的基因和差异甲基化的基因之间没有重叠,表明沃尔巴克氏菌-感染相关的DNA甲基化变化不会直接调节基因表达的水平。此外,受这些机制影响的基因表现出独特的进化历史。由于感染而差异甲基化的基因趋向于保守。相比之下,差异表达的基因则更可能是赤眼蜂属谱系所独有的,表明宿主对感染的转录反应。尽管如此,我们发现了沃尔巴赫菌相关DNA甲基化变化的几个新颖方面。甲基化差异基因包括那些参与卵母细胞发育和染色体分离的基因。有趣的是,Wolbachia感染与更高水平的DNA甲基化有关。另外,即使考虑到DNA甲基化的影响,Wolbachia感染也降低了基因表达的总体变异性。我们还确定了特定的案例,其中外显子替代使用与Wolbachia感染引起的DNA甲基化变化有关。这些结果开始揭示经受沃尔巴克菌诱导的表观遗传修饰和/或宿主对沃尔巴克菌感染的不同基因和分子途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号