当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Homogeneous activation of peroxymonosulfate using a low-dosage cross-bridged cyclam manganese(II) complex for organic pollutant degradation via a nonradical pathway.
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jhazmat.2020.122560 Yuqing Yu 1 , Peng Tan 2 , Xinjue Huang 1 , Junjie Tao 1 , Yingying Liu 3 , Raymond Jianxiong Zeng 1 , Man Chen 1 , Shungui Zhou 1
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jhazmat.2020.122560 Yuqing Yu 1 , Peng Tan 2 , Xinjue Huang 1 , Junjie Tao 1 , Yingying Liu 3 , Raymond Jianxiong Zeng 1 , Man Chen 1 , Shungui Zhou 1
Affiliation
|
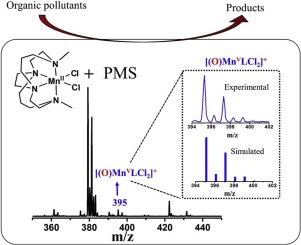
The high dosage of catalyst requirement and weak anti-interference ability limit current heterogeneous manganese (Mn) catalyst/peroxymonosulfate (PMS) systems to remediate the organic polluted wastewater in complicated environment. Inspired by the concept of atom economy, herein, a homogenous manganese complex bearing a cross-bridged cyclam ligand Mn(cbc)Cl2 (MnL, L = cbc = 4,11-dimethyl-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane)) is capable of activating PMS for reactive brilliant red K-2BP (RBR K-2BP) degradation. The dosage of MnL for PMS activation was low, in a range of 0.38∼3.8 mg/L. The quenching experiments demonstrated that the degradation was a nonradical-controlled process. Using methyl phenyl sulfoxide (PMSO) as a probe, the dominated degradation process of substrate was via an oxygen transfer pathway. Moreover, a high-valent Mn-oxo [(O)MnVLCl2]+ was directly detected using electrospray ionization mass spectrometry (ESI/MS). This system showed excellent anti-interference ability to both anions and humic acid, a typical natural organic matter. The atom economy, represented by an index ((mg pollutant)/h/(g catalyst)), showed that MnL 22737 in PMS activation was much higher than those of Mn-based heterogeneous catalytic systems 67∼960 and was only behind that of iron-tetraamidomacrocyclic ligand Fe-TAML 59139. This work provides insights into designing an atom-economic Mn-based PMS activator for efficient treatments for organic pollutants in a complicated environment.
中文翻译:

使用低剂量交叉桥环仙客来锰(II)配合物通过非自由基途径降解过氧单硫酸盐的均相活化。
高剂量的催化剂需求和较弱的抗干扰能力限制了目前的多相锰(Mn)催化剂/过氧单硫酸盐(PMS)系统在复杂环境中修复有机污染废水的能力。受原子经济概念的启发,本文中的均相锰配合物带有交叉桥联的Cyclam配体Mn(cbc)Cl2(MnL,L = cbc = 4,11-二甲基-1,4,8,11-四氮杂双环[6.6] .2]十六烷))能够活化PMS进行活性艳红K-2BP(RBR K-2BP)降解。用于PMS活化的MnL剂量较低,范围为0.38〜3.8 mg / L。淬灭实验表明降解是非自由基控制的过程。使用甲基苯基亚砜(PMSO)作为探针,底物的主要降解过程是通过氧转移途径。此外,使用电喷雾电离质谱法(ESI / MS)直接检测高价Mn-氧代[(O)MnVLCl2] +。该系统对阴离子和腐殖酸(一种典型的天然有机物)均具有出色的抗干扰能力。用指数((mg污染物)/ h /(g催化剂))表示的原子经济性表明,MnS 22737在PMS活化中远高于Mn基非均相催化体系67〜960,并且仅落后于铁-四酰胺基大环配体Fe-TAML59139。这项工作为设计一种原子经济的锰基PMS活化剂提供了见识,该活化剂可有效处理复杂环境中的有机污染物。典型的天然有机物。用指数((mg污染物)/ h /(g催化剂))表示的原子经济性表明,MnS 22737在PMS活化中远高于Mn基非均相催化体系67〜960,并且仅落后于铁-四酰胺基大环配体Fe-TAML59139。这项工作为设计一种原子经济的锰基PMS活化剂提供了见识,该活化剂可有效处理复杂环境中的有机污染物。典型的天然有机物。用指数((mg污染物)/ h /(g催化剂))表示的原子经济性表明,MnS 22737在PMS活化中远高于Mn基非均相催化体系67〜960,并且仅落后于铁-四酰胺基大环配体Fe-TAML59139。这项工作为设计一种原子经济的锰基PMS活化剂提供了见识,该活化剂可有效处理复杂环境中的有机污染物。
更新日期:2020-03-19
中文翻译:

使用低剂量交叉桥环仙客来锰(II)配合物通过非自由基途径降解过氧单硫酸盐的均相活化。
高剂量的催化剂需求和较弱的抗干扰能力限制了目前的多相锰(Mn)催化剂/过氧单硫酸盐(PMS)系统在复杂环境中修复有机污染废水的能力。受原子经济概念的启发,本文中的均相锰配合物带有交叉桥联的Cyclam配体Mn(cbc)Cl2(MnL,L = cbc = 4,11-二甲基-1,4,8,11-四氮杂双环[6.6] .2]十六烷))能够活化PMS进行活性艳红K-2BP(RBR K-2BP)降解。用于PMS活化的MnL剂量较低,范围为0.38〜3.8 mg / L。淬灭实验表明降解是非自由基控制的过程。使用甲基苯基亚砜(PMSO)作为探针,底物的主要降解过程是通过氧转移途径。此外,使用电喷雾电离质谱法(ESI / MS)直接检测高价Mn-氧代[(O)MnVLCl2] +。该系统对阴离子和腐殖酸(一种典型的天然有机物)均具有出色的抗干扰能力。用指数((mg污染物)/ h /(g催化剂))表示的原子经济性表明,MnS 22737在PMS活化中远高于Mn基非均相催化体系67〜960,并且仅落后于铁-四酰胺基大环配体Fe-TAML59139。这项工作为设计一种原子经济的锰基PMS活化剂提供了见识,该活化剂可有效处理复杂环境中的有机污染物。典型的天然有机物。用指数((mg污染物)/ h /(g催化剂))表示的原子经济性表明,MnS 22737在PMS活化中远高于Mn基非均相催化体系67〜960,并且仅落后于铁-四酰胺基大环配体Fe-TAML59139。这项工作为设计一种原子经济的锰基PMS活化剂提供了见识,该活化剂可有效处理复杂环境中的有机污染物。典型的天然有机物。用指数((mg污染物)/ h /(g催化剂))表示的原子经济性表明,MnS 22737在PMS活化中远高于Mn基非均相催化体系67〜960,并且仅落后于铁-四酰胺基大环配体Fe-TAML59139。这项工作为设计一种原子经济的锰基PMS活化剂提供了见识,该活化剂可有效处理复杂环境中的有机污染物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号