Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jmb.2020.03.016 Brandon R Goblirsch 1 , Michael C Wiener 1
|
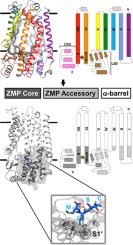
Ste24, an integral membrane protein zinc metalloprotease, is found in every kingdom of eukaryotes. It was discovered approximately 20 years ago by yeast genetic screens identifying it as a factor responsible for processing the yeast mating a-factor pheromone. In animals, Ste24 processes prelamin A, a component of the nuclear lamina; mutations in the human ortholog of Ste24 diminish its activity, giving rise to genetic diseases of accelerated aging (progerias). Additionally, lipodystrophy, acquired from the standard highly active antiretroviral therapy used to treat AIDS patients, likely results from off-target interactions of HIV (aspartyl) protease inhibitor drugs with Ste24. Ste24 possesses a novel “α-barrel” structure, consisting of a ring of seven transmembrane α-helices enclosing a large (> 12,000 Å3) interior volume that contains the active-site and substrate-binding region; this “membrane-interior reaction chamber” is unprecedented in integral membrane protein structures. Additionally, the surface of the membrane-interior reaction chamber possesses a strikingly large negative electrostatic surface potential, adding additional “functional mystery.” Recent publications implicate Ste24 as a key factor in several endoplasmic reticulum processes, including the unfolded protein response, a cellular stress response of the endoplasmic reticulum, and removal of misfolded proteins from the translocon. Ste24, with its provocative structure, enigmatic mechanism, and recently emergent new biological roles including “translocon unclogger” and (non-enyzmatic) broad-spectrum viral restriction factor, presents far differently than before 2016, when it was viewed as a “CAAX protease” responsible for cleavage of prenylated (farnesylated or geranylgeranylated) substrates. The emphasis of this review is on Ste24 of the “Post-CAAX-Protease Era.”
中文翻译:

Ste24:具有激发结构和新兴生物学的整体膜蛋白锌金属蛋白酶。
在每个真核生物王国中都发现了Ste24,一种必不可少的膜蛋白锌金属蛋白酶。大约20年前,通过酵母遗传筛选发现了它,并将其确定为负责处理与酵母-信息素交配的因素。在动物体内,Ste24处理核纤层蛋白preprein A;Ste24的人类直系同源基因中的突变会减弱其活性,从而导致遗传性疾病加速衰老(早衰)。此外,从用于治疗AIDS患者的标准高效抗逆转录病毒疗法中获得的脂肪营养不良可能是HIV(天冬氨酰)蛋白酶抑制剂药物与Ste24脱靶相互作用的结果。Ste24具有新颖的“α桶”结构,由七个大跨度(> 12,000Å)的跨膜α螺旋环组成3)包含活性位点和底物结合区的内部体积;这种“膜内反应室”在完整的膜蛋白结构中是前所未有的。此外,膜内部反应室的表面具有惊人的大负静电表面电势,从而增加了其他“功能奥秘”。最近的出版物暗示Ste24是几个内质网过程的关键因素,包括未折叠的蛋白质反应,内质网的细胞应激反应以及从转运子中去除错误折叠的蛋白质。Ste24具有挑衅性的结构,令人迷惑的机制以及最近崭露头角的新生物学角色,包括“ translocon unclogger”和(非酶促)广谱病毒限制因子,其呈现方式与2016年之前大不相同,当它被认为是“ CAAX蛋白酶”时,负责裂解烯丙基化(法呢基化或香叶基香叶基化)底物。本文的重点是“后CAAX蛋白酶时代”的Ste24。











































 京公网安备 11010802027423号
京公网安备 11010802027423号