当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Local Self-Enhancement of MinD Membrane Binding in Min Protein Pattern Formation.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jmb.2020.03.012 Tamara Heermann 1 , Beatrice Ramm 1 , Samson Glaser 1 , Petra Schwille 1
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.jmb.2020.03.012 Tamara Heermann 1 , Beatrice Ramm 1 , Samson Glaser 1 , Petra Schwille 1
Affiliation
|
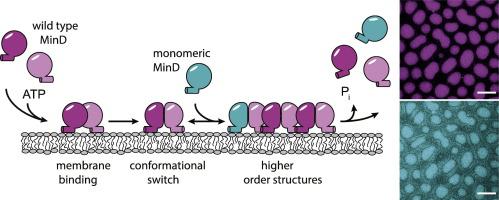
The proteins MinD, MinE and MinC are constitutive for the spatiotemporal organization of cell division in Escherichia coli, in particular, for positioning the division machinery at mid-cell. To achieve this function, the ATPase MinD and the ATPase-activating protein MinE undergo coordinated pole-to-pole oscillations and have thus become a paradigm for protein pattern formation in biology. The exact molecular mechanisms enabling MinDE self-organization, and particularly the role of cooperativity in the membrane binding of MinD, thought to be a key requirement, have remained poorly understood. However, for bottom-up synthetic biology aiming at a de novo design of key cellular features, elucidating these mechanisms is of great relevance. By combining in vitro reconstitution with rationally guided mutagenesis of MinD, we found that when bound to membranes, MinD displays new interfaces for multimerization, which are distinct from the canonical MinD dimerization site. We propose that these additional transient interactions contribute to the local self-enhancement of MinD at the membrane, while their relative lability maintains the structural plasticity required for MinDE wave propagation. This could represent a powerful structural regulation feature not reported so far for self-organizing proteins.
中文翻译:

MinD膜结合在Min蛋白模式形成中的局部自我增强。
MinD,MinE和MinC蛋白质对于大肠杆菌中细胞分裂的时空组织是组成性的,特别是在细胞中部定位分裂机制。为了实现此功能,ATPase MinD和ATPase激活蛋白MinE经历了协调的极对极振动,因此成为生物学中蛋白质模式形成的范例。使MinDE自组织的确切分子机制,尤其是协同作用在MinD的膜结合中的作用(被认为是关键要求),仍然知之甚少。然而,对于旨在从头开始对关键细胞特征进行重新设计的自下而上的合成生物学而言,阐明这些机制具有重大意义。通过将体外重建与合理指导的MinD诱变相结合,我们发现当与膜结合时,MinD显示出新的多聚界面,这与规范的MinD二聚位点不同。我们建议这些额外的瞬态相互作用有助于MinD在膜上的局部自我增强,而它们的相对不稳定性则保持了MinDE波传播所需的结构可塑性。这可能代表了迄今为止尚未报道的自组织蛋白的强大结构调控功能。
更新日期:2020-03-19
中文翻译:

MinD膜结合在Min蛋白模式形成中的局部自我增强。
MinD,MinE和MinC蛋白质对于大肠杆菌中细胞分裂的时空组织是组成性的,特别是在细胞中部定位分裂机制。为了实现此功能,ATPase MinD和ATPase激活蛋白MinE经历了协调的极对极振动,因此成为生物学中蛋白质模式形成的范例。使MinDE自组织的确切分子机制,尤其是协同作用在MinD的膜结合中的作用(被认为是关键要求),仍然知之甚少。然而,对于旨在从头开始对关键细胞特征进行重新设计的自下而上的合成生物学而言,阐明这些机制具有重大意义。通过将体外重建与合理指导的MinD诱变相结合,我们发现当与膜结合时,MinD显示出新的多聚界面,这与规范的MinD二聚位点不同。我们建议这些额外的瞬态相互作用有助于MinD在膜上的局部自我增强,而它们的相对不稳定性则保持了MinDE波传播所需的结构可塑性。这可能代表了迄今为止尚未报道的自组织蛋白的强大结构调控功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号