当前位置:
X-MOL 学术
›
Adv. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New view of the adsorption of surfactants at water/alkane interfaces - Competitive and cooperative effects of surfactant and alkane molecules.
Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.cis.2020.102143 V B Fainerman 1 , E V Aksenenko 2 , V I Kovalchuk 3 , N Mucic 4 , A Javadi 5 , L Liggieri 6 , F Ravera 6 , G Loglio 6 , A V Makievski 1 , E Schneck 7 , R Miller 7
Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.cis.2020.102143 V B Fainerman 1 , E V Aksenenko 2 , V I Kovalchuk 3 , N Mucic 4 , A Javadi 5 , L Liggieri 6 , F Ravera 6 , G Loglio 6 , A V Makievski 1 , E Schneck 7 , R Miller 7
Affiliation
|
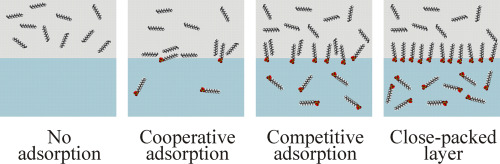
The theoretical description of the adsorption of surfactants at interfaces between aqueous solutions and oil was based over a very long time on models derived for the solution/air interface. Thus, most of the experimentally observed peculiarities could not be specifically considered but were merely interpreted in terms of a penetration of oil molecules into the alkyl chain layer of the adsorbed surfactant molecules. These penetrating oil molecules enhance the surfactant adsorption as compared to the water/air interface. Later on, for the special situations at water/oil interfaces a competitive adsorption of surfactant and oil molecules was postulated, allowing a much better description of experimental data. This picture, however, was unable to explain why the interfacial tension of the water/oil interface decreases very quickly when extremely small amounts of surfactants are added to the water. This effect cannot be of competitive nature, but a cooperativity of surfactant and oil molecules forming a mixed adsorption layer is required instead. This cooperative effect means that already few surfactant molecules adsorbed at the interface can induce a significant ordering of oil molecules in the interfacial layer. This new interfacial structure, in turn, attracts further surfactant molecules to adsorb. Improving the theoretical description of experimental data was finally achieved by applying suitable adsorption models for the two adsorbing compounds, i.e. a Frumkin adsorption model for the oil molecules and a Langmuir, Frumkin, or reorientation model for the adsorbing surfactant molecules. Here, the progress in modelling surfactant adsorption at water/oil interfaces is discussed mainly for the homologous series of the cationic surfactants CnTAB, of the anionic surfactant SDS, and members of the homologous series of the non-ionic surfactants CnDMPO at water/alkane interfaces.
中文翻译:

表面活性剂在水/烷烃界面上吸附的新观点-表面活性剂和烷烃分子的竞争和协同作用。
表面活性剂在水溶液和油之间的界面上的吸附的理论描述是基于很长时间以来基于溶液/空气界面的模型的。因此,大多数实验观察到的特性不能具体考虑,而仅是根据油分子渗透到吸附的表面活性剂分子的烷基链层中来解释。与水/空气界面相比,这些渗透性油分子增强了表面活性剂的吸附。后来,针对水/油界面的特殊情况,假定了表面活性剂和油分子的竞争性吸附,从而可以更好地描述实验数据。但是这张照片 不能解释为什么当向水中添加极少量的表面活性剂时,水/油界面的界面张力会迅速降低。该效果不能具有竞争性,但是需要表面活性剂和形成混合吸附层的油分子的协同作用。这种协同作用意味着已经很少有吸附在界面上的表面活性剂分子可以在界面层中引起油分子的显着排序。反过来,这种新的界面结构吸引了更多的表面活性剂分子进行吸附。最终,通过对两种吸附化合物应用合适的吸附模型,即对油分子的Frumkin吸附模型和Langmuir,Frumkin,或吸附表面活性剂分子的重新定向模型。在此,主要针对阳离子表面活性剂CnTAB的同源系列,阴离子表面活性剂SDS以及水/烷烃界面的非离子表面活性剂CnDMPO同源系列的成员,讨论了在水/油界面上吸附表面活性剂的建模进展。 。
更新日期:2020-03-19
中文翻译:

表面活性剂在水/烷烃界面上吸附的新观点-表面活性剂和烷烃分子的竞争和协同作用。
表面活性剂在水溶液和油之间的界面上的吸附的理论描述是基于很长时间以来基于溶液/空气界面的模型的。因此,大多数实验观察到的特性不能具体考虑,而仅是根据油分子渗透到吸附的表面活性剂分子的烷基链层中来解释。与水/空气界面相比,这些渗透性油分子增强了表面活性剂的吸附。后来,针对水/油界面的特殊情况,假定了表面活性剂和油分子的竞争性吸附,从而可以更好地描述实验数据。但是这张照片 不能解释为什么当向水中添加极少量的表面活性剂时,水/油界面的界面张力会迅速降低。该效果不能具有竞争性,但是需要表面活性剂和形成混合吸附层的油分子的协同作用。这种协同作用意味着已经很少有吸附在界面上的表面活性剂分子可以在界面层中引起油分子的显着排序。反过来,这种新的界面结构吸引了更多的表面活性剂分子进行吸附。最终,通过对两种吸附化合物应用合适的吸附模型,即对油分子的Frumkin吸附模型和Langmuir,Frumkin,或吸附表面活性剂分子的重新定向模型。在此,主要针对阳离子表面活性剂CnTAB的同源系列,阴离子表面活性剂SDS以及水/烷烃界面的非离子表面活性剂CnDMPO同源系列的成员,讨论了在水/油界面上吸附表面活性剂的建模进展。 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号