当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric gemini surfactants modified vermiculite- and silica nanosheets-based adsorbents for removing methyl orange and crystal violet
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.colsurfa.2020.124735 Gaili Cao , Manglai Gao , Tao Shen , Shangxin Guo , Bingbing Zhao , Qing Zhao
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.colsurfa.2020.124735 Gaili Cao , Manglai Gao , Tao Shen , Shangxin Guo , Bingbing Zhao , Qing Zhao
|
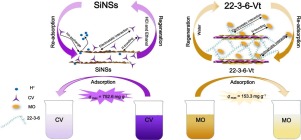
Abstract Vermiculite- and silica nanosheets-based adsorbents have received widespread attention due to the advantages of low cost, high efficiency, easy preparation and regeneration. In this study, vermiculite (Vt), silica nanosheets (SiNSs), organo-vermiculites (OVts) and organo-silica nanosheets (OSiNSs) modified by a series of asymmetric gemini surfactants [C22H45(CH3)2N+(CH2)sN+(CH3)2C6H13]Br2 (s is the spacer length, s = 2, 3, 6) were selected as adsorbents to study their adsorption behavior for removing cationic crystal violet (CV) and anionic methyl orange (MO) dyes. The structures and surface characters of adsorbents were revealed by various characterization techniques such as FT-IR, XRD, TG-DTG, SEM and EDS. From the results of adsorption experiments, SiNSs exhibited conspicuous adsorption towards cationic CV over Vt, OVts and OSiNSs, nevertheless, the adsorption capacity of 22-3-6-Vt for MO was superior to that of Vt, 22-2-6-Vt, 22-6-6-Vt, SiNSs and OSiNSs, which can be ascribed to the type of precursors, structures of adsorbents and dyes, charge characteristics of dye molecules and spacer length of the surfactants. qmax of CV on SiNSs reached 752.6 mg g−1 and that of MO onto 22-3-6-Vt attained 153.3 mg g−1. Furthermore, kinetics, isotherms and thermodynamics were executed for exploring retention mechanisms. The spent 22-3-6-Vt and SiNSs were regenerated by water and a mixture of 0.1 mol L−1 HCl solution and ethanol, respectively.
中文翻译:

用于去除甲基橙和结晶紫的不对称双子表面活性剂改性蛭石和二氧化硅纳米片基吸附剂
摘要 蛭石和二氧化硅纳米片基吸附剂由于具有低成本、高效、易于制备和再生等优点而受到广泛关注。在这项研究中,蛭石 (Vt)、二氧化硅纳米片 (SiNSs)、有机蛭石 (OVts) 和有机二氧化硅纳米片 (OSiNSs) 由一系列不对称双子表面活性剂 [C22H45(CH3)2N+(CH2)sN+(CH3)选择2C6H13]Br2(s是间隔长度,s = 2, 3, 6)作为吸附剂,研究其去除阳离子结晶紫(CV)和阴离子甲基橙(MO)染料的吸附行为。通过FT-IR、XRD、TG-DTG、SEM和EDS等多种表征技术揭示了吸附剂的结构和表面特征。从吸附实验的结果来看,SiNSs 对 Vt、OVts 和 OSiNSs 的阳离子 CV 表现出明显的吸附,尽管如此,22-3-6-Vt 对 MO 的吸附能力优于 Vt、22-2-6-Vt、22-6-6-Vt、SiNSs 和 OSiNSs,这可以归因于前体、吸附剂和染料的结构、染料分子的电荷特性和表面活性剂的间隔长度。SiNS 上 CV 的 qmax 达到 752.6 mg g-1,而 MO 在 22-3-6-Vt 上的 qmax 达到 153.3 mg g-1。此外,执行动力学、等温线和热力学以探索保留机制。用过的 22-3-6-Vt 和 SiNSs 分别用水和 0.1 mol L-1 HCl 溶液和乙醇的混合物再生。染料分子的电荷特性和表面活性剂的间隔长度。SiNS 上 CV 的 qmax 达到 752.6 mg g-1,而 MO 在 22-3-6-Vt 上的 qmax 达到 153.3 mg g-1。此外,执行动力学、等温线和热力学以探索保留机制。用过的 22-3-6-Vt 和 SiNSs 分别用水和 0.1 mol L-1 HCl 溶液和乙醇的混合物再生。染料分子的电荷特性和表面活性剂的间隔长度。SiNS 上 CV 的 qmax 达到 752.6 mg g-1,而 MO 在 22-3-6-Vt 上的 qmax 达到 153.3 mg g-1。此外,执行动力学、等温线和热力学以探索保留机制。用过的 22-3-6-Vt 和 SiNSs 分别用水和 0.1 mol L-1 HCl 溶液和乙醇的混合物再生。
更新日期:2020-07-01
中文翻译:

用于去除甲基橙和结晶紫的不对称双子表面活性剂改性蛭石和二氧化硅纳米片基吸附剂
摘要 蛭石和二氧化硅纳米片基吸附剂由于具有低成本、高效、易于制备和再生等优点而受到广泛关注。在这项研究中,蛭石 (Vt)、二氧化硅纳米片 (SiNSs)、有机蛭石 (OVts) 和有机二氧化硅纳米片 (OSiNSs) 由一系列不对称双子表面活性剂 [C22H45(CH3)2N+(CH2)sN+(CH3)选择2C6H13]Br2(s是间隔长度,s = 2, 3, 6)作为吸附剂,研究其去除阳离子结晶紫(CV)和阴离子甲基橙(MO)染料的吸附行为。通过FT-IR、XRD、TG-DTG、SEM和EDS等多种表征技术揭示了吸附剂的结构和表面特征。从吸附实验的结果来看,SiNSs 对 Vt、OVts 和 OSiNSs 的阳离子 CV 表现出明显的吸附,尽管如此,22-3-6-Vt 对 MO 的吸附能力优于 Vt、22-2-6-Vt、22-6-6-Vt、SiNSs 和 OSiNSs,这可以归因于前体、吸附剂和染料的结构、染料分子的电荷特性和表面活性剂的间隔长度。SiNS 上 CV 的 qmax 达到 752.6 mg g-1,而 MO 在 22-3-6-Vt 上的 qmax 达到 153.3 mg g-1。此外,执行动力学、等温线和热力学以探索保留机制。用过的 22-3-6-Vt 和 SiNSs 分别用水和 0.1 mol L-1 HCl 溶液和乙醇的混合物再生。染料分子的电荷特性和表面活性剂的间隔长度。SiNS 上 CV 的 qmax 达到 752.6 mg g-1,而 MO 在 22-3-6-Vt 上的 qmax 达到 153.3 mg g-1。此外,执行动力学、等温线和热力学以探索保留机制。用过的 22-3-6-Vt 和 SiNSs 分别用水和 0.1 mol L-1 HCl 溶液和乙醇的混合物再生。染料分子的电荷特性和表面活性剂的间隔长度。SiNS 上 CV 的 qmax 达到 752.6 mg g-1,而 MO 在 22-3-6-Vt 上的 qmax 达到 153.3 mg g-1。此外,执行动力学、等温线和热力学以探索保留机制。用过的 22-3-6-Vt 和 SiNSs 分别用水和 0.1 mol L-1 HCl 溶液和乙醇的混合物再生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号