Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.bmc.2020.115437 Huifang Guo , Kai Cheng , Yan Gao , Weiqi Bai , Cai Wu , Wei He , Conggang Li , Zhuorong Li
|
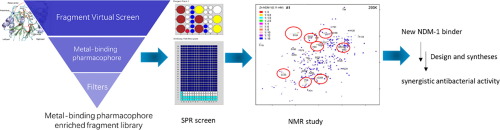
NDM-1 can hydrolyze nearly all available β-lactam antibiotics, including carbapenems. NDM-1 producing bacterial strains are worldwide threats. It is still very challenging to find a potent NDM-1 inhibitor for clinical use. In our study, we used a metal-binding pharmacophore (MBP) enriched virtual fragment library to screen NDM-1 hits. SPR screening helped to verify the MBP virtual hits and identified a new NDM-1 binder and weak inhibitor A1. A solution NMR study of 15N-labeled NDM-1 showed that A1 disturbed all three residues coordinating the second zinc ion (Zn2) in the active pocket of NDM-1. The perturbation only happened in the presence of zinc ion, indicating that A1 bound to Zn2. Based on the scaffold of A1, we designed and synthesized a series of NDM-1 inhibitors. Several compounds showed synergistic antibacterial activity with meropenem against NDM-1 producing K. pneumoniae.
中文翻译:

通过片段虚拟,SPR和NMR筛选鉴定了一种新型的强金属结合NDM-1抑制剂
NDM-1可以水解几乎所有可用的β-内酰胺抗生素,包括碳青霉烯。产生NDM-1的细菌菌株是全世界的威胁。寻找一种有效的NDM-1抑制剂用于临床仍是非常具有挑战性的。在我们的研究中,我们使用了富含金属结合药效团(MBP)的虚拟片段库来筛选NDM-1命中。SPR筛选有助于验证MBP虚拟命中,并鉴定了新的NDM-1结合剂和弱抑制剂A1。一项对15个N标记的NDM-1的溶液NMR研究表明,A1干扰了NDM-1活性口袋中与第二个锌离子(Zn2)配位的所有三个残基。摄动仅在锌离子存在下发生,表明A1绑定到Zn2。基于A1的支架,我们设计并合成了一系列NDM-1抑制剂。几种化合物与美罗培南对产生NDM-1的肺炎克雷伯菌显示协同抗菌活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号