当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemically Generated KO2 as a Phase-Transfer Mediator for Na–O2 Batteries
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acs.jpcc.9b11631 Min Soo Jung 1 , Seongmin Ha 1 , Dongho Koo 1 , Kyu Tae Lee 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acs.jpcc.9b11631 Min Soo Jung 1 , Seongmin Ha 1 , Dongho Koo 1 , Kyu Tae Lee 1
Affiliation
|
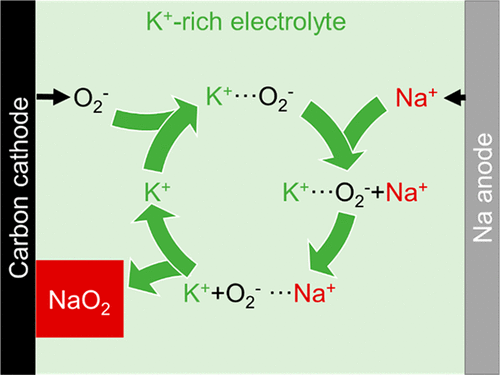
Superoxide-based Na–O2 batteries have attracted much attention as promising alternatives to peroxide-based Li–O2 batteries because of their small polarization for oxygen evolution reactions. However, the limited solubility of their discharge product, NaO2, leads to the surface-confined mechanism at high current densities, resulting in the poor energy density of Na–O2 batteries. In this connection, a few protic phase-transfer catalysts, such as water and benzoic acid, have been examined to improve reversible capacity because they promote the solution-mediated mechanism. Herein, KO2, which is electrochemically generated from potassium trifluoromethanesulfonate dissolved in electrolytes during discharge, is introduced as a phase-transfer mediator for Na–O2 batteries. The reaction mechanism of Na–O2 batteries containing a KO2 mediator is clarified through ex situ XRD, cross-sectional SEM, and ICP analyses. KO2 plays the role of a phase-transfer mediator because the desolvation rate of KO2 is slower than that of NaO2. As a result, Na–O2 batteries with KO2 show the solution-mediated mechanism rather than the surface-confined mechanism, thus delivering a high reversible capacity of approximately 6 mAh cm–2. In addition, since KO2 is chemically and electrochemically more stable than previous protic phase-transfer mediators, Na–O2 cells with KO2 show stable cycle performance, such as negligible capacity fading over 25 cycles.
中文翻译:

电化学生成的KO 2作为Na–O 2电池的相转移介体
基于超氧化物的Na–O 2电池作为基于过氧化物的Li–O 2电池的有前途的替代品已经引起了广泛的关注,因为它们的氧释放反应极化很小。但是,它们的放电产物NaO 2的溶解度有限,导致在高电流密度下的表面受限机制,从而导致Na–O 2电池的能量密度很低。在这方面,已经研究了一些质子相转移催化剂,例如水和苯甲酸,以提高可逆容量,因为它们促进了溶液介导的机理。KO 2它是由放电过程中溶解在电解质中的三氟甲磺酸钾电化学生成的,被引入作为Na-O 2电池的相转移介质。通过异位XRD,横截面SEM和ICP分析,阐明了含有KO 2介体的Na–O 2电池的反应机理。KO 2起到相转移介体的作用,因为KO 2的去溶剂化速度比NaO 2慢。结果,带有KO 2的Na–O 2电池显示出溶液介导的机理,而不是表面受限的机理,因此可提供约6 mAh cm的高可逆容量。–2。另外,由于KO 2在化学和电化学上比以前的质子相转移介体更稳定,因此带有KO 2的Na–O 2细胞表现出稳定的循环性能,例如在25个循环中可忽略不计的容量。
更新日期:2020-03-27
中文翻译:

电化学生成的KO 2作为Na–O 2电池的相转移介体
基于超氧化物的Na–O 2电池作为基于过氧化物的Li–O 2电池的有前途的替代品已经引起了广泛的关注,因为它们的氧释放反应极化很小。但是,它们的放电产物NaO 2的溶解度有限,导致在高电流密度下的表面受限机制,从而导致Na–O 2电池的能量密度很低。在这方面,已经研究了一些质子相转移催化剂,例如水和苯甲酸,以提高可逆容量,因为它们促进了溶液介导的机理。KO 2它是由放电过程中溶解在电解质中的三氟甲磺酸钾电化学生成的,被引入作为Na-O 2电池的相转移介质。通过异位XRD,横截面SEM和ICP分析,阐明了含有KO 2介体的Na–O 2电池的反应机理。KO 2起到相转移介体的作用,因为KO 2的去溶剂化速度比NaO 2慢。结果,带有KO 2的Na–O 2电池显示出溶液介导的机理,而不是表面受限的机理,因此可提供约6 mAh cm的高可逆容量。–2。另外,由于KO 2在化学和电化学上比以前的质子相转移介体更稳定,因此带有KO 2的Na–O 2细胞表现出稳定的循环性能,例如在25个循环中可忽略不计的容量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号