当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
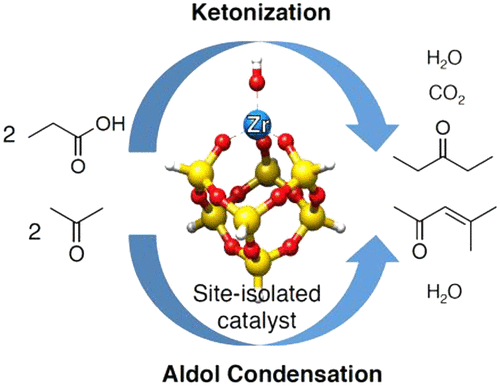
Experimental and Computational Studies of Carbon–Carbon Bond Formation via Ketonization and Aldol Condensation over Site-Isolated Zirconium Catalysts
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-18 , DOI: 10.1021/acscatal.9b05176 Sankaranarayanapillai Shylesh 1 , Lance A. Bettinson 1, 2 , Ahmed Aljahri 1 , Martin Head-Gordon 1, 3 , Alexis T. Bell 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-18 , DOI: 10.1021/acscatal.9b05176 Sankaranarayanapillai Shylesh 1 , Lance A. Bettinson 1, 2 , Ahmed Aljahri 1 , Martin Head-Gordon 1, 3 , Alexis T. Bell 1, 2
Affiliation
|
We report here the preparation and investigation of isolated Zr centers supported on a high surface area silica for the conversion of carboxylic acids to internal ketones by ketonic decarboxylation (ketonization) and the aldol condensation of ketones to dimeric enones. Catalysts were synthesized by the grafting of Cp2ZrCl2 on the surface of amorphous silica. The connectivity of Zr was characterized by XRD, UV–vis, and Raman spectroscopy. For the lowest Zr loading, Zr is present predominantly as isolated monomeric species. As the Zr loading is increased, a progressively larger fraction of Zr forms oligomeric species and ZrO2 nanoparticles. Measurements of catalytic activity show that the turnover frequency for carboxylic acid ketonic decarboxylation reaction and aldol condensation of ketones decreases monotonically with increasing Zr loading. An H/D kinetic isotope effect was not observed over isolated Zr catalysts, suggesting that α-H abstraction is not the rate-determining step, rather C–C bond forming may be rate limiting for both reactions. This conclusion is supported by computational modeling of the reaction mechanism. The proposed catalytic cycle for ketonization proceeds via a β-keto acid intermediate on isolated Zr sites that are always coordinatively saturated with C–C bond formation as the rate-limiting step. C–C bond formation is also rate-determining for aldol condensation, with an apparent activation energy that is in good agreement with the experiment if the resting state is a saturated ≡ZrOH site with two adsorbed ketone molecules.
中文翻译:

现场隔离的锆催化剂上酮化和醛醇缩合形成碳-碳键的实验和计算研究
我们在这里报告制备和研究分离的Zr中心,该中心在高表面积二氧化硅上负载,用于通过酮基脱羧(酮化)和酮与二聚烯酮的醛醇缩合将羧酸转化为内酮。通过将Cp 2 ZrCl 2接枝到无定形二氧化硅表面上来合成催化剂。Zr的连通性通过XRD,UV-vis和拉曼光谱表征。对于最低的Zr负载,Zr主要以孤立的单体形式存在。随着Zr负载的增加,Zr的份额逐渐增加,形成寡聚物种和ZrO 2纳米粒子。催化活性的测量结果表明,随着Zr负载量的增加,羧酸酮的脱羧反应和酮的醛醇缩合的反应频率降低。在孤立的Zr催化剂上未观察到H / D动力学同位素效应,这表明α-H的提取不是决定速率的步骤,而是C-C键的形成可能限制了这两个反应的速率。该结论得到反应机理的计算模型的支持。拟议的酮化催化循环是通过β-酮酸中间体在孤立的Zr位点上进行的,该位点始终与C–C键的形成协调地饱和,作为限速步骤。C–C键的形成也决定了醛醇缩合的速率,
更新日期:2020-04-23
中文翻译:

现场隔离的锆催化剂上酮化和醛醇缩合形成碳-碳键的实验和计算研究
我们在这里报告制备和研究分离的Zr中心,该中心在高表面积二氧化硅上负载,用于通过酮基脱羧(酮化)和酮与二聚烯酮的醛醇缩合将羧酸转化为内酮。通过将Cp 2 ZrCl 2接枝到无定形二氧化硅表面上来合成催化剂。Zr的连通性通过XRD,UV-vis和拉曼光谱表征。对于最低的Zr负载,Zr主要以孤立的单体形式存在。随着Zr负载的增加,Zr的份额逐渐增加,形成寡聚物种和ZrO 2纳米粒子。催化活性的测量结果表明,随着Zr负载量的增加,羧酸酮的脱羧反应和酮的醛醇缩合的反应频率降低。在孤立的Zr催化剂上未观察到H / D动力学同位素效应,这表明α-H的提取不是决定速率的步骤,而是C-C键的形成可能限制了这两个反应的速率。该结论得到反应机理的计算模型的支持。拟议的酮化催化循环是通过β-酮酸中间体在孤立的Zr位点上进行的,该位点始终与C–C键的形成协调地饱和,作为限速步骤。C–C键的形成也决定了醛醇缩合的速率,



























 京公网安备 11010802027423号
京公网安备 11010802027423号