Laboratory Investigation ( IF 5.1 ) Pub Date : 2020-03-18 , DOI: 10.1038/s41374-020-0415-6 Sophie Sarah Steinhaeuser 1 , Erika Morera 1 , Zuzana Budkova 1 , Alexander Schepsky 1 , Qiong Wang 2 , Ottar Rolfsson 2 , Angela Riedel 3, 4 , Aileen Krueger 3, 4 , Bylgja Hilmarsdottir 5 , Gunhild Mari Maelandsmo 5 , Bryndis Valdimarsdottir 1 , Anna Karen Sigurdardottir 1 , Bjarni Agnar Agnarsson 6, 7 , Jon Gunnlaugur Jonasson 6, 7 , Saevar Ingthorsson 1 , Gunnhildur Asta Traustadottir 1 , Thordur Oskarsson 3, 4, 8 , Thorarinn Gudjonsson 1, 2, 9
|
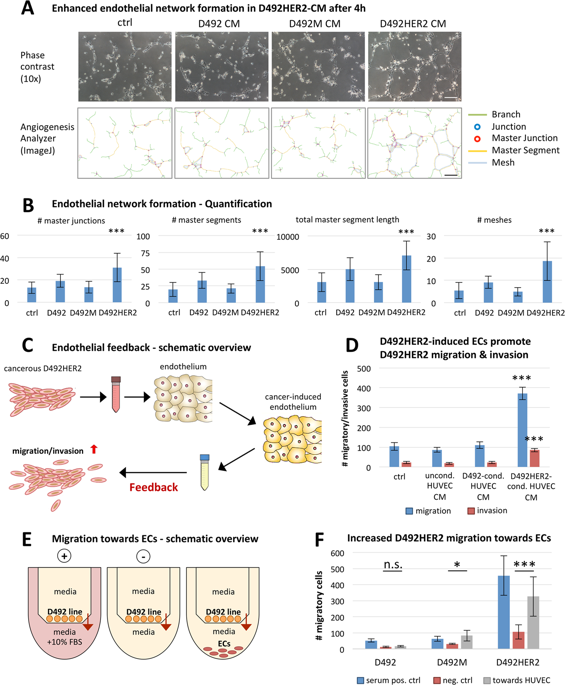
The tumor microenvironment is increasingly recognized as key player in cancer progression. Investigating heterotypic interactions between cancer cells and their microenvironment is important for understanding how specific cell types support cancer. Forming the vasculature, endothelial cells (ECs) are a prominent cell type in the microenvironment of both normal and neoplastic breast gland. Here, we sought out to analyze epithelial–endothelial cross talk in the breast using isogenic non-tumorigenic vs. tumorigenic breast epithelial cell lines and primary ECs. The cellular model used here consists of D492, a breast epithelial cell line with stem cell properties, and two isogenic D492-derived EMT cell lines, D492M and D492HER2. D492M was generated by endothelial-induced EMT and is non-tumorigenic while D492HER2 is tumorigenic, expressing the ErbB2/HER2 oncogene. To investigate cellular cross talk, we used both conditioned medium (CM) and 2D/3D co-culture systems. Secretome analysis of D492 cell lines was performed using mass spectrometry and candidate knockdown (KD), and overexpression (OE) was done using siRNA and CRISPRi/CRISPRa technology. D492HER2 directly enhances endothelial network formation and activates a molecular axis in ECs promoting D492HER2 migration and invasion, suggesting an endothelial feedback response. Secretome analysis identified extracellular matrix protein 1 (ECM1) as potential angiogenic inducer in D492HER2. Confirming its involvement, KD of ECM1 reduced the ability of D492HER2-CM to increase endothelial network formation and induce the endothelial feedback, while recombinant ECM1 (rECM1) increased both. Interestingly, NOTCH1 and NOTCH3 expression was upregulated in ECs upon treatment with D492HER2-CM or rECM1 but not by CM from D492HER2 with ECM1 KD. Blocking endothelial NOTCH signaling inhibited the increase in network formation and the ability of ECs to promote D492HER2 migration and invasion. In summary, our data demonstrate that cancer-secreted ECM1 induces a NOTCH-mediated endothelial feedback promoting cancer progression by enhancing migration and invasion. Targeting this interaction may provide a novel possibility to improve cancer treatment.
中文翻译:

HER2 过表达乳腺癌细胞分泌的 ECM1 促进血管生态位的形成,加速癌细胞迁移和侵袭。
肿瘤微环境越来越被认为是癌症进展的关键因素。研究癌细胞与其微环境之间的异型相互作用对于了解特定细胞类型如何支持癌症非常重要。形成脉管系统的内皮细胞 (EC) 是正常和肿瘤性乳腺微环境中的重要细胞类型。在这里,我们试图使用同基因非致瘤性与致瘤性乳腺上皮细胞系和原代 EC 来分析乳腺中的上皮-内皮串扰。此处使用的细胞模型由 D492(一种具有干细胞特性的乳腺上皮细胞系)和两个同基因 D492 衍生的 EMT 细胞系 D492M 和 D492HER2 组成。D492M 由内皮诱导的 EMT 产生并且是非致瘤性的,而 D492HER2 是致瘤性的,表达 ErbB2/HER2 致癌基因。为了研究细胞串扰,我们使用了条件培养基 (CM) 和 2D/3D 共培养系统。使用质谱法和候选基因敲低 (KD) 对 D492 细胞系进行分泌蛋白组分析,并使用 siRNA 和 CRISPRi/CRISPRa 技术进行过表达 (OE)。D492HER2 直接增强内皮网络形成并激活 EC 中促进 D492HER2 迁移和侵袭的分子轴,表明存在内皮反馈反应。分泌蛋白组分析将细胞外基质蛋白 1 (ECM1) 鉴定为 D492HER2 中潜在的血管生成诱导剂。确认其参与,ECM1 的 KD 降低了 D492HER2-CM 增加内皮网络形成和诱导内皮反馈的能力,而重组 ECM1 (rECM1) 增加了两者。有趣的是,在用 D492HER2-CM 或 rECM1 处理后,ECs 中的 NOTCH1 和 NOTCH3 表达上调,但来自 D492HER2 的 CM 和 ECM1 KD 没有上调。阻断内皮 NOTCH 信号传导抑制了网络形成的增加和 EC 促进 D492HER2 迁移和侵袭的能力。总之,我们的数据表明,癌症分泌的 ECM1 诱导 NOTCH 介导的内皮反馈,通过增强迁移和侵袭促进癌症进展。针对这种相互作用可能会为改善癌症治疗提供一种新的可能性。我们的数据表明,癌症分泌的 ECM1 诱导 NOTCH 介导的内皮反馈,通过增强迁移和侵袭促进癌症进展。针对这种相互作用可能会为改善癌症治疗提供一种新的可能性。我们的数据表明,癌症分泌的 ECM1 诱导 NOTCH 介导的内皮反馈,通过增强迁移和侵袭促进癌症进展。针对这种相互作用可能会为改善癌症治疗提供一种新的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号