Journal of Nuclear Materials ( IF 2.8 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.jnucmat.2020.152095 Jinfan Chen , Zhong Long , Ruizhi Qiu , Yin Hu , Bingyun Ao , Kezhao Liu
|
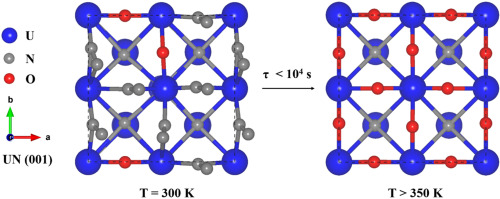
Density functional theory calculations were performed to study reactions on uranium mononitride (UN) surface when it is in contact with environment gases such as O2, H2O, N2, H2. The chemical adsorption of O2 on UN surface is much more active than other surface reactions. Microkinetic analysis shows that the UN surface is mainly adsorbed by N2 at room temperature, suggesting nitrogen molecules in the air can protect UN from being oxidized. When temperature is above 350 K, the rate of surface oxidation speeds up significantly with the full O-adsorption on UN occurring within a relatively short time. Ab initio thermodynamic calculations display that the UN surface undergoes atomic rearrangements upon oxygen adsorption, which leads to the expansion of crystal lattice and opens the pathways for O diffusion into the sub-surface. Our work constitutes the first ab initio-based kinetic study of surface reactions on UN and contributes to the understanding of oxidation corrosion of this promising nuclear fuel at atomic scales.
中文翻译:

DFT研究表明,UN(001)表面上暴露于环境气体中的分子反应和氧化腐蚀:
进行了密度泛函理论计算,以研究单氮化铀(UN)与环境气体(如O 2,H 2 O,N 2,H 2)接触时的反应。O 2在UN表面的化学吸附比其他表面反应要活跃得多。微观动力学分析表明,UN表面主要被N 2吸附在室温下,表明空气中的氮分子可以保护联合国免受氧化。当温度高于350 K时,表面氧化速率会显着加快,同时在相对较短的时间内UN会完全吸附O。从头算热力学计算表明,UN表面在吸附氧后会发生原子重排,这导致晶格膨胀,并为O扩散到亚表面中打开了路径。我们的工作是对联合国表面反应从头开始的从头开始的动力学研究,并且有助于理解这种有前途的核燃料在原子尺度上的氧化腐蚀。











































 京公网安备 11010802027423号
京公网安备 11010802027423号