Biomaterials Advances ( IF 7.9 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.msec.2020.110857 Soumya Saroj 1 , Devi Sirisha Janni 1 , Chandrasekhar Reddy Ummadi 1 , Muraleedharan Kannoth Manheri 1
|
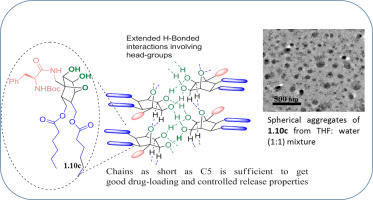
A new group of non-ionic amphiphiles with short alkyl chains and functionalizable oxanorbornane-based head group for drug delivery application are presented. They can be prepared through a sequence that starts with cycloaddition of Boc-protected furfuryl amine with maleic anhydride and reduction of the resulting adduct with LiAlH4 to get a diol intermediate. Introduction of alkyl chains through these primary hydroxyl groups and subsequent head-group modification via cis-hydroxylation resulted in a number of new amphiphiles in good yields. They were characterized by various spectro-analytical techniques and then subjected to drug-delivery studies using ibuprofen as a model drug. Functionalization of the head group through the amine functionality was also done with an intention to improve lipid packing to get better drug-loading and release properties. Irrespective of the nature of groups attached through this amine unit, all amphiphiles with short alkyl chains were found to assemble into spherical aggregates when drop-casted from various organic solvents. The same assembly preference prevailed in their formulations containing lipid-cholesterol-drug in 1: 0.5:1 ratio as well, and these particles had diameters <300 nm. Apart from good drug-loading efficiencies, these amphiphiles exhibited controlled release properties and did not show any indication of toxicity when assayed against NIH3T3 cells. The formulation based on lipid having a phenylalanine unit on the head group (1.10c) turned out to be the best in this series which showed a loading efficiency of 57.6% with a controlled release of ~42% by end of 24 h. Because of efficient layering that is facilitated by hydrogen bonding involving well-directed hydroxyl groups on the head group, amphiphiles with alkyl chains as short as C5 are able to act as efficient drug delivery systems, which is one of the highlights of this work.
中文翻译:

新型非离子两亲物的设计中基于功能性氧杂硼烷的头基及其药物传递特性。
提出了一组新的具有短烷基链的非离子两亲物和可官能化的基于氧杂硼烷的头基,用于药物递送。它们可以通过以下顺序制备:从Boc保护的糠胺与马来酸酐的环加成开始,然后用LiAlH 4还原生成的加合物得到二醇中间体。通过这些伯羟基引入烷基链并随后通过顺式羟基化修饰头部,从而以高收率产生了许多新的两亲物。通过各种光谱分析技术对其进行了表征,然后使用布洛芬作为模型药物进行了药物传递研究。还通过胺官能团对头基进行了官能化,目的是改善脂质堆积以获得更好的载药量和释放性能。无论通过该胺单元连接的基团的性质如何,当从各种有机溶剂中滴铸时,发现所有具有短烷基链的两亲物都组装成球形聚集体。相同比例的装配中也普遍使用比例为1:0.5:1的脂质-胆固醇-药物的制剂,这些颗粒的直径小于300 nm。除了良好的载药效率外,这些两亲物还表现出控释特性,并且在针对NIH3T3细胞进行测定时未显示任何毒性迹象。基于在头基上具有苯丙氨酸单元的脂质的制剂(1.10c)是该系列中最好的,在24小时结束时显示出57.6%的装载效率,并有42%的受控释放。由于氢键促进了有效的分层,氢键涉及头基上的良好定向的羟基,因此具有短至C5烷基链的两亲物能够充当有效的药物递送系统,这是这项工作的重点之一。



























 京公网安备 11010802027423号
京公网安备 11010802027423号