Reactive & Functional Polymers ( IF 4.5 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.reactfunctpolym.2020.104570 Nadia H. Elsayed , Ayshah Alatawi , M. Monier
|
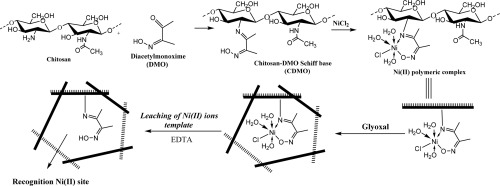
An ion-imprinted sorbent (Ni-CDMO) derived from diacetylmonoxime (DMO)-chitosan Schiff base has been prepared by incorporating Ni(II) ion matching sites, which can selectively coordinate and recover Ni(II) ions from aquatic media. The chitosan was first modified by DMO to improve the targeted Ni(II) ion coordination and then the polymeric Ni-complex was treated with glyoxal to cross-link the polysaccharide chains and maintain the coordination sites rigid and inflexible after eluting the Ni(II) ions from the sorbent matrix. The maximum Ni(II) ion capacities of the Ni-CDMO and the control adsorbent C-CDMO were determined by performing the isotherm studies under different Ni(II) initial concentrations and treating the obtained experimental results using Langmuir and Freundlich models. The maximum capacity was around 135 mg/g, which is considered a high competing value. Furthermore, the competitive extraction of Ni(II) ions among various similar ions including Pb(II), Cu(II), Co(II), and Cd(II) was carried out using Ni-CDMO and C-CDMO and the results confirmed the distinct role of the utilized imprinting procedure in creating a considerable Ni(II) ion selectivity within the structure of the Ni-CDMO. Also, the regeneration and reusability experiments indicated the preservation of approximately 98% of the initial efficiency after the performance of five consecutive cycles.
中文翻译:

二乙酰基单氧杂环丁烷改性的壳聚糖衍生的离子印迹聚合物,用于选择性固相萃取镍(II)离子
通过并入Ni(II)离子匹配位点,制备了可从二乙酰基单肟(DMO)-壳聚糖席夫碱衍生的离子印迹吸附剂(Ni-CDMO),该位点可以选择性地协调和从水介质中回收Ni(II)离子。首先通过DMO改性壳聚糖以改善目标Ni(II)离子的配位,然后用乙二醛处理聚合的Ni-络合物以使多糖链交联并洗脱Ni(II)后保持配位位点的刚性和刚性吸附剂基质中的离子。通过在不同的Ni(II)初始浓度下进行等温线研究并使用Langmuir和Freundlich模型处理获得的实验结果,确定Ni-CDMO和对照吸附剂C-CDMO的最大Ni(II)离子容量。最大容量约为135 mg / g,这被认为是很高的竞争价值。此外,使用Ni-CDMO和C-CDMO对包括Pb(II),Cu(II),Co(II)和Cd(II)在内的各种相似离子中的Ni(II)离子进行竞争性萃取。证实了利用压印程序在Ni-CDMO结构内产生可观的Ni(II)离子选择性方面的独特作用。同样,再生和可重复使用性实验表明,连续执行五个循环后,可以保留大约98%的初始效率。Cd(II)是使用Ni-CDMO和C-CDMO进行的,结果证实了所用的压印程序在Ni-CDMO结构内产生相当大的Ni(II)离子选择性方面的独特作用。同样,再生和可重复使用性实验表明,在执行了五个连续循环后,保留了约98%的初始效率。Cd(II)是使用Ni-CDMO和C-CDMO进行的,结果证实了所用的压印程序在Ni-CDMO结构内产生相当大的Ni(II)离子选择性方面的独特作用。同样,再生和可重复使用性实验表明,连续执行五个循环后,可以保留大约98%的初始效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号