当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bimetallic ions regulate pore size and chemistry of zeolites for selective adsorption of ethylene from ethane
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.ces.2020.115636 Yuzong Liu , Ying Wu , Wanwen Liang , Junjie Peng , Zhong Li , Haihui Wang , Michael J. Janik , Jing Xiao
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.ces.2020.115636 Yuzong Liu , Ying Wu , Wanwen Liang , Junjie Peng , Zhong Li , Haihui Wang , Michael J. Janik , Jing Xiao
|
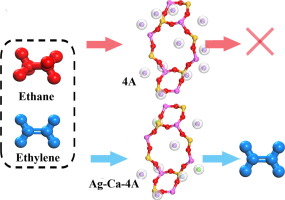
Abstract The petrochemical industry currently accomplishes olefin/paraffin separation by energy-intensive cryogenic distillation at an enormous scale. We report a sequential Ca2+/Ag+ ion-exchanged zeolite that achieves nearly ideal molecular sieving of C2H4/C2H6 and superior C2H4 adsorption capacity. Sequential and partial ion exchange regulates the pore size in ±0.2 A increments, ranging between 3.8 and 4.2 A. The demonstrated C2H4 adsorption capacity of 3.7 mmol/g, under ambient conditions, is the highest among zeolite-based materials. Elaborated with DFT calculations, Ag+-induced the stretching of the C-H bond and reduction of H-C-H bond angle of the C2H4 molecule in confined pore, providing C2H4 with the molecular basis and favorable kinetics for selective admission to pore size even less than 4 A. The strategy of using bimetallic ions to regulate pore aperture size and selective admission of gas molecules with favorable kinetics provides a general path to be extended to other analogous molecular separation processes.
中文翻译:

双金属离子调节沸石的孔径和化学性质,用于从乙烷中选择性吸附乙烯
摘要 目前,石油化工行业通过高耗能的低温精馏实现了烯烃/烷烃的大规模分离。我们报告了连续 Ca2+/Ag+ 离子交换沸石,它实现了近乎理想的 C2H4/C2H6 分子筛分和卓越的 C2H4 吸附能力。顺序和部分离子交换以 ±0.2 A 的增量调节孔径,范围在 3.8 和 4.2 A 之间。在环境条件下,所证明的 C2H4 吸附容量为 3.7 mmol/g,是沸石基材料中最高的。通过 DFT 计算,Ag+ 在受限孔隙中诱导了 CH 键的拉伸和 C2H4 分子的 HCH 键角的减小,为 C2H4 提供了分子基础和有利的动力学,以选择性地进入小于 4 A 的孔径。
更新日期:2020-07-01
中文翻译:

双金属离子调节沸石的孔径和化学性质,用于从乙烷中选择性吸附乙烯
摘要 目前,石油化工行业通过高耗能的低温精馏实现了烯烃/烷烃的大规模分离。我们报告了连续 Ca2+/Ag+ 离子交换沸石,它实现了近乎理想的 C2H4/C2H6 分子筛分和卓越的 C2H4 吸附能力。顺序和部分离子交换以 ±0.2 A 的增量调节孔径,范围在 3.8 和 4.2 A 之间。在环境条件下,所证明的 C2H4 吸附容量为 3.7 mmol/g,是沸石基材料中最高的。通过 DFT 计算,Ag+ 在受限孔隙中诱导了 CH 键的拉伸和 C2H4 分子的 HCH 键角的减小,为 C2H4 提供了分子基础和有利的动力学,以选择性地进入小于 4 A 的孔径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号