当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Selective Histone Deacetylase 6 (HDAC6) Degraders Recruiting Von Hippel-Lindau (VHL) E3 Ubiquitin Ligase.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-18 , DOI: 10.1021/acsmedchemlett.0c00046 Ka Yang 1 , Hao Wu 1 , Zhongrui Zhang 2 , Eric D Leisten 1 , Xueqing Nie 1 , Binkai Liu 1 , Zhi Wen 3 , Jing Zhang 3 , Michael D Cunningham 1 , Weiping Tang 1, 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-18 , DOI: 10.1021/acsmedchemlett.0c00046 Ka Yang 1 , Hao Wu 1 , Zhongrui Zhang 2 , Eric D Leisten 1 , Xueqing Nie 1 , Binkai Liu 1 , Zhi Wen 3 , Jing Zhang 3 , Michael D Cunningham 1 , Weiping Tang 1, 2
Affiliation
|
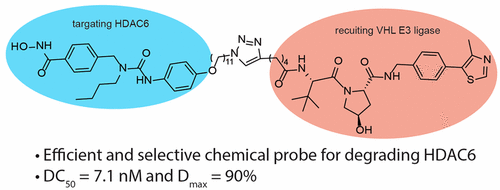
Histone deacetylase 6 (HDAC6) is involved in multiple cellular processes such as aggresome formation, protein stability, and cell motility. Numerous HDAC6-selective inhibitors have been developed as cellular chemical tools to elucidate the function of HDAC6. Since HDAC6 has multiple domains that cannot be studied by HDAC6-selective inhibitors, CRISPR-CAS9 and siRNA/shRNA have been employed to elucidate the nonenzymatic functions of HDAC6. However, these genetic methods have many limitations. Proteolysis targeting chimera (PROTAC) is an emerging technology for the development of small molecules that can quickly remove the entire protein in cells. We previously developed multifunctional HDAC6 degraders that can recruit cereblon (CRBN) E3 ubiquitin ligase. These HDAC6 degraders can degrade not only HDAC6 but also neo-substrates of CRBN. They are excellent candidates for the development of anticancer therapeutics, but the multifunctional nature of the CRBN-based HDAC6 degraders has limited their utility as specific chemical probes for the study of HDAC6-related cellular pathways. Herein we report the development of the first cell-permeable HDAC6-selective degraders employing Von Hippel-Lindau (VHL) E3 ubiquitin ligase, which does not have any known neo-substrates. The DC50's of the most potent compound 3j are 7.1 nM and 4.3 nM in human MM1S and mouse 4935 cell lines, respectively. The D max's of 3j in these two cell lines are 90% and 57%, respectively.
中文翻译:

招募Von Hippel-Lindau(VHL)E3泛素连接酶的选择性组蛋白脱乙酰基酶6(HDAC6)降解剂的开发。
组蛋白脱乙酰基酶6(HDAC6)参与多种细胞过程,如聚集体形成,蛋白质稳定性和细胞运动性。已经开发出许多HDAC6选择性抑制剂作为细胞化学工具来阐明HDAC6的功能。由于HDAC6具有无法通过HDAC6选择性抑制剂研究的多个域,因此CRISPR-CAS9和siRNA / shRNA已被用于阐明HDAC6的非酶功能。但是,这些遗传方法有许多局限性。靶向嵌合体的蛋白水解(PROTAC)是一种新兴的技术,用于开发可以迅速去除细胞中整个蛋白质的小分子。我们以前开发了可以募集大脑(CRBN)E3泛素连接酶的多功能HDAC6降解剂。这些HDAC6降解剂不仅可以降解HDAC6,而且可以降解CRBN的新底物。它们是开发抗癌疗法的极佳候选者,但是基于CRBN的HDAC6降解子的多功能性质限制了它们作为研究HDAC6相关细胞途径的特定化学探针的用途。本文中,我们报道了使用Von Hippel-Lindau(VHL)E3泛素连接酶开发的首个可渗透细胞的HDAC6选择性降解剂,该酶没有任何已知的新底物。在人的MM1S和小鼠4935细胞系中,最有效的化合物3j的DC50分别为7.1 nM和4.3 nM。在这两个细胞系中3j的D max分别为90%和57%。但是基于CRBN的HDAC6降解子的多功能性质限制了其作为研究HDAC6相关细胞途径的特定化学探针的用途。本文中,我们报道了使用Von Hippel-Lindau(VHL)E3泛素连接酶开发的首个可渗透细胞的HDAC6选择性降解剂,该酶没有任何已知的新底物。在人的MM1S和小鼠4935细胞系中,最有效的化合物3j的DC50分别为7.1 nM和4.3 nM。在这两个细胞系中3j的D max分别为90%和57%。但是基于CRBN的HDAC6降解子的多功能性质限制了其作为研究HDAC6相关细胞途径的特定化学探针的用途。本文中,我们报道了使用Von Hippel-Lindau(VHL)E3泛素连接酶开发的首个可渗透细胞的HDAC6选择性降解剂,该酶没有任何已知的新底物。在人的MM1S和小鼠4935细胞系中,最有效的化合物3j的DC50分别为7.1 nM和4.3 nM。在这两个细胞系中3j的D max分别为90%和57%。人MM1S和小鼠4935细胞株分别为3 nM。在这两个细胞系中3j的D max分别为90%和57%。人MM1S和小鼠4935细胞株分别为3 nM。在这两个细胞系中3j的D max分别为90%和57%。
更新日期:2020-04-23
中文翻译:

招募Von Hippel-Lindau(VHL)E3泛素连接酶的选择性组蛋白脱乙酰基酶6(HDAC6)降解剂的开发。
组蛋白脱乙酰基酶6(HDAC6)参与多种细胞过程,如聚集体形成,蛋白质稳定性和细胞运动性。已经开发出许多HDAC6选择性抑制剂作为细胞化学工具来阐明HDAC6的功能。由于HDAC6具有无法通过HDAC6选择性抑制剂研究的多个域,因此CRISPR-CAS9和siRNA / shRNA已被用于阐明HDAC6的非酶功能。但是,这些遗传方法有许多局限性。靶向嵌合体的蛋白水解(PROTAC)是一种新兴的技术,用于开发可以迅速去除细胞中整个蛋白质的小分子。我们以前开发了可以募集大脑(CRBN)E3泛素连接酶的多功能HDAC6降解剂。这些HDAC6降解剂不仅可以降解HDAC6,而且可以降解CRBN的新底物。它们是开发抗癌疗法的极佳候选者,但是基于CRBN的HDAC6降解子的多功能性质限制了它们作为研究HDAC6相关细胞途径的特定化学探针的用途。本文中,我们报道了使用Von Hippel-Lindau(VHL)E3泛素连接酶开发的首个可渗透细胞的HDAC6选择性降解剂,该酶没有任何已知的新底物。在人的MM1S和小鼠4935细胞系中,最有效的化合物3j的DC50分别为7.1 nM和4.3 nM。在这两个细胞系中3j的D max分别为90%和57%。但是基于CRBN的HDAC6降解子的多功能性质限制了其作为研究HDAC6相关细胞途径的特定化学探针的用途。本文中,我们报道了使用Von Hippel-Lindau(VHL)E3泛素连接酶开发的首个可渗透细胞的HDAC6选择性降解剂,该酶没有任何已知的新底物。在人的MM1S和小鼠4935细胞系中,最有效的化合物3j的DC50分别为7.1 nM和4.3 nM。在这两个细胞系中3j的D max分别为90%和57%。但是基于CRBN的HDAC6降解子的多功能性质限制了其作为研究HDAC6相关细胞途径的特定化学探针的用途。本文中,我们报道了使用Von Hippel-Lindau(VHL)E3泛素连接酶开发的首个可渗透细胞的HDAC6选择性降解剂,该酶没有任何已知的新底物。在人的MM1S和小鼠4935细胞系中,最有效的化合物3j的DC50分别为7.1 nM和4.3 nM。在这两个细胞系中3j的D max分别为90%和57%。人MM1S和小鼠4935细胞株分别为3 nM。在这两个细胞系中3j的D max分别为90%和57%。人MM1S和小鼠4935细胞株分别为3 nM。在这两个细胞系中3j的D max分别为90%和57%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号