当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemically Prepared Li0.6FePO4 Solid Solution as a Vehicle for Studying Phase Separation Kinetics in Li-Ion Battery Materials
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jpcc.9b09279 Laurence Savignac 1 , John M. Griffin 2, 3 , Steen B. Schougaard 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.jpcc.9b09279 Laurence Savignac 1 , John M. Griffin 2, 3 , Steen B. Schougaard 1
Affiliation
|
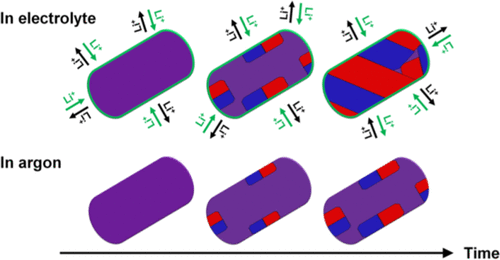
The commercial success of LiFePO4 in high-power Li-ion batteries is strongly related to its unique ultrahigh-rate charge/discharge performance that permits full charge in less than a minute. Since Li1–xFePO4 (0.05 ≤ x ≤ 0.95) separates into two phases with poor electronic and ionic conduction, this raises questions regarding the structural dynamics of phase separation. In this paper, the transformation of metastable solid solution Li0.6FePO4 into a phase-separated material is studied by analysis of the local and bulk structure. 6Li MAS NMR is used to probe the immediate environment where proximity to Fe3+ results in a significant shift in resonance frequency. Conversely, time-resolved X-ray diffraction (XRD) measurements reveal the transformation kinetics at the unit cell scale. The XRD showed no preferential relaxation along the a, b, and c crystal axes, consistent with the absence of a phase boundary perpendicular to the fast diffusion b axis. Key to the analysis is the preparation of the solid solution, which yields phase-pure samples exhibiting no evidence of the thermodynamically stable LiFePO4 or FePO4 phases. Long-term measurement indicated that after 263 days under an argon atmosphere these samples still exhibited a solid solution fraction > 40%. However, in the presence of an electrolyte, phase separation is significantly more rapid. The results presented support Li et al. model [Nat. Mater.2018, 17, 915], where vehicular lithium transport at the surface determines the rate of phase separation and offers a methodology for studying high-energy-density LiMPO4 systems (M = transition metal) that currently are limited by poor high-rate performance.
中文翻译:

化学制备的Li 0.6 FePO 4固溶体作为研究锂离子电池材料中相分离动力学的载体
LiFePO 4在高功率锂离子电池中的商业成功与它独特的超高速率充电/放电性能密切相关,该性能允许在不到一分钟的时间内完成充电。由于Li 1- X的FePO 4(0.05≤ X ≤0.95)分离成两个相具有差的电子和离子传导,这提出了关于相分离的结构动力学的问题。本文通过分析局部和本体结构,研究了亚稳固溶体Li 0.6 FePO 4向相分离材料的转化。6 Li MAS NMR用于探测邻近Fe 3+的直接环境导致谐振频率发生明显变化。相反,时间分辨X射线衍射(XRD)测量揭示了单位晶格尺度上的转化动力学。XRD沿a,b和c晶轴未显示优先弛豫,这与不存在垂直于快速扩散b轴的相界一致。分析的关键是固溶体的制备,它产生的纯相样品没有热力学稳定的LiFePO 4或FePO 4的证据。阶段。长期测量表明,在氩气气氛下放置263天后,这些样品的固溶度仍大于40%。然而,在电解质的存在下,相分离明显更快。结果提供了支持李等。模特[ Nat。母校 2018,17,915],其中,车辆用锂运输在表面处确定的相分离,并提供用于研究的高能量密度的LiMPO的方法的速率4个系统(M =过渡金属),目前由差的高速性能的限制。
更新日期:2020-03-30
中文翻译:

化学制备的Li 0.6 FePO 4固溶体作为研究锂离子电池材料中相分离动力学的载体
LiFePO 4在高功率锂离子电池中的商业成功与它独特的超高速率充电/放电性能密切相关,该性能允许在不到一分钟的时间内完成充电。由于Li 1- X的FePO 4(0.05≤ X ≤0.95)分离成两个相具有差的电子和离子传导,这提出了关于相分离的结构动力学的问题。本文通过分析局部和本体结构,研究了亚稳固溶体Li 0.6 FePO 4向相分离材料的转化。6 Li MAS NMR用于探测邻近Fe 3+的直接环境导致谐振频率发生明显变化。相反,时间分辨X射线衍射(XRD)测量揭示了单位晶格尺度上的转化动力学。XRD沿a,b和c晶轴未显示优先弛豫,这与不存在垂直于快速扩散b轴的相界一致。分析的关键是固溶体的制备,它产生的纯相样品没有热力学稳定的LiFePO 4或FePO 4的证据。阶段。长期测量表明,在氩气气氛下放置263天后,这些样品的固溶度仍大于40%。然而,在电解质的存在下,相分离明显更快。结果提供了支持李等。模特[ Nat。母校 2018,17,915],其中,车辆用锂运输在表面处确定的相分离,并提供用于研究的高能量密度的LiMPO的方法的速率4个系统(M =过渡金属),目前由差的高速性能的限制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号