当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt/Peracetic Acid: Advanced Oxidation of Aromatic Organic Compounds by Acetylperoxyl Radicals.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.est.0c00356 Juhee Kim 1 , Penghui Du 2 , Wen Liu 3 , Cong Luo 1 , He Zhao 2 , Ching-Hua Huang 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.est.0c00356 Juhee Kim 1 , Penghui Du 2 , Wen Liu 3 , Cong Luo 1 , He Zhao 2 , Ching-Hua Huang 1
Affiliation
|
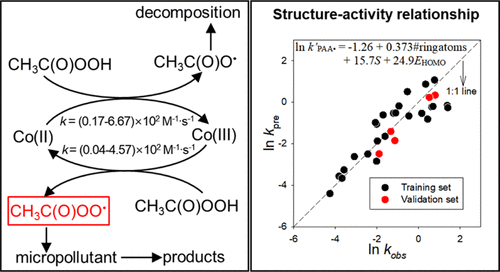
Peracetic acid (PAA) is increasingly used as an alternative disinfectant and its advanced oxidation processes (AOPs) could be useful for pollutant degradation. Co(II) or Co(III) can activate PAA to produce acetyloxyl (CH3C(O)O•) and acetylperoxyl (CH3C(O)OO•) radicals with little •OH radical formation, and Co(II)/Co(III) is cycled. For the first time, this study determined the reaction rates of PAA with Co(II) (kPAA,Co(II) = 1.70 × 101 to 6.67 × 102 M-1·s-1) and Co(III) (kPAA,Co(III) = 3.91 × 100 to 4.57 × 102 M-1·s-1) ions over the initial pH 3.0-8.2 and evaluated 30 different aromatic organic compounds for degradation by Co/PAA. In-depth investigation confirmed that CH3C(O)OO• is the key reactive species under Co/PAA for compound degradation. Assessing the structure-activity relationship between compounds' molecular descriptors and pseudo-first-order degradation rate constants (k'PAA• in s-1) by Co/PAA showed the number of ring atoms, EHOMO, softness, and ionization potential to be the most influential, strongly suggesting the electron transfer mechanism from aromatic compounds to the acetylperoxyl radical. The radical production and compound degradation in Co/PAA are most efficient in the intermediate pH range and can be influenced by water matrix constituents of bicarbonate, phosphate, and humic acids. These results significantly improve the knowledge regarding the acetylperoxyl radical from PAA and will be useful for further development and applications of PAA-based AOPs.
中文翻译:

钴/过氧乙酸:乙酰基过氧自由基对芳香族有机化合物的高级氧化。
过氧乙酸(PAA)越来越多地用作替代消毒剂,其先进的氧化过程(AOP)可能对污染物的降解有用。Co(II)或Co(III)可以活化PAA产生几乎没有•OH自由基形成的乙酰氧基(CH3C(O)O•)和乙酰过氧基(CH3C(O)OO•)自由基,以及Co(II)/ Co(III) )循环。这项研究首次确定了PAA与Co(II)(kPAA,Co(II)= 1.70×101至6.67×102 M-1·s-1)和Co(III)(kPAA,Co (III)在初始pH 3.0-8.2时为3.91×100至4.57×102 M-1·s-1)个离子,并评估了30种不同的芳香族有机化合物被Co / PAA降解的情况。深入研究证实,CH3C(O)OO•是Co / PAA下化合物降解的关键反应物种。评估化合物之间的构效关系 Co / PAA的分子描述符和伪一级降解速率常数(s-1中的k'PAA•)显示,环原子数,EHOMO,柔软度和电离势最有影响力,强烈暗示了电子转移芳香化合物到乙酰基过氧自由基的机理。Co / PAA中自由基的产生和化合物的降解在中等pH范围内最有效,并且会受到碳酸氢盐,磷酸盐和腐殖酸的水基质成分的影响。这些结果大大改善了有关PAA乙酰过氧自由基的知识,将对基于PAA的AOP的进一步开发和应用有用。有力地暗示了从芳族化合物到乙酰基过氧自由基的电子转移机理。Co / PAA中自由基的产生和化合物的降解在中等pH范围内最有效,并且会受到碳酸氢盐,磷酸盐和腐殖酸的水基质成分的影响。这些结果大大改善了有关PAA的乙酰过氧自由基的知识,将对基于PAA的AOP的进一步开发和应用有用。有力地暗示了从芳族化合物到乙酰基过氧自由基的电子转移机理。Co / PAA中自由基的产生和化合物的降解在中等pH范围内最有效,并且会受到碳酸氢盐,磷酸盐和腐殖酸的水基质成分的影响。这些结果大大改善了有关PAA乙酰过氧自由基的知识,将对基于PAA的AOP的进一步开发和应用有用。
更新日期:2020-04-23
中文翻译:

钴/过氧乙酸:乙酰基过氧自由基对芳香族有机化合物的高级氧化。
过氧乙酸(PAA)越来越多地用作替代消毒剂,其先进的氧化过程(AOP)可能对污染物的降解有用。Co(II)或Co(III)可以活化PAA产生几乎没有•OH自由基形成的乙酰氧基(CH3C(O)O•)和乙酰过氧基(CH3C(O)OO•)自由基,以及Co(II)/ Co(III) )循环。这项研究首次确定了PAA与Co(II)(kPAA,Co(II)= 1.70×101至6.67×102 M-1·s-1)和Co(III)(kPAA,Co (III)在初始pH 3.0-8.2时为3.91×100至4.57×102 M-1·s-1)个离子,并评估了30种不同的芳香族有机化合物被Co / PAA降解的情况。深入研究证实,CH3C(O)OO•是Co / PAA下化合物降解的关键反应物种。评估化合物之间的构效关系 Co / PAA的分子描述符和伪一级降解速率常数(s-1中的k'PAA•)显示,环原子数,EHOMO,柔软度和电离势最有影响力,强烈暗示了电子转移芳香化合物到乙酰基过氧自由基的机理。Co / PAA中自由基的产生和化合物的降解在中等pH范围内最有效,并且会受到碳酸氢盐,磷酸盐和腐殖酸的水基质成分的影响。这些结果大大改善了有关PAA乙酰过氧自由基的知识,将对基于PAA的AOP的进一步开发和应用有用。有力地暗示了从芳族化合物到乙酰基过氧自由基的电子转移机理。Co / PAA中自由基的产生和化合物的降解在中等pH范围内最有效,并且会受到碳酸氢盐,磷酸盐和腐殖酸的水基质成分的影响。这些结果大大改善了有关PAA的乙酰过氧自由基的知识,将对基于PAA的AOP的进一步开发和应用有用。有力地暗示了从芳族化合物到乙酰基过氧自由基的电子转移机理。Co / PAA中自由基的产生和化合物的降解在中等pH范围内最有效,并且会受到碳酸氢盐,磷酸盐和腐殖酸的水基质成分的影响。这些结果大大改善了有关PAA乙酰过氧自由基的知识,将对基于PAA的AOP的进一步开发和应用有用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号