当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crown Ether-Functionalized Polybenzoxazine for Metal Ion Adsorption
Macromolecules ( IF 5.1 ) Pub Date : 2020-03-18 , DOI: 10.1021/acs.macromol.9b02519 Mohamed Gamal Mohamed, Shiao-Wei Kuo
Macromolecules ( IF 5.1 ) Pub Date : 2020-03-18 , DOI: 10.1021/acs.macromol.9b02519 Mohamed Gamal Mohamed, Shiao-Wei Kuo
|
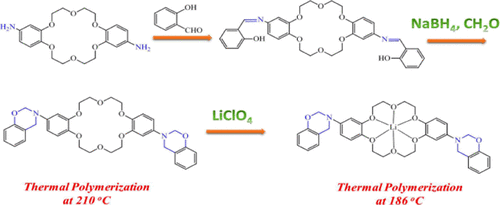
In this study, we synthesized a new crown ether-functionalized benzoxazine monomer (crown-ether BZ) in high yield and purity through reduction of the Schiff base prepared from a dibenzo[18]crown-6 diamine derivative and salicylaldehyde and subsequent reaction of the resulting o-hydroxybenzylamine species with CH2O. We used differential scanning calorimetry (DSC), Fourier transform infrared (FTIR) spectroscopy, and thermogravimetric analysis to examine the thermal ring opening polymerization and thermal stability of the crown-ether BZ monomer during various types of thermal treatment. DSC revealed that this crown-ether BZ monomer featured a relatively low curing temperature (210 °C; that of the typical Pa-type 3-phenyl-3,4-dihydro-2H-benzooxazine monomer: 263 °C) because the flexibility of the crown ether moiety on the main chain backbone structure catalyzed the ring opening polymerization. We also used DSC, FTIR spectroscopy, and ionic conductivity measurements to investigate the specific metal–crown ether interactions of crown-ether BZ/LiClO4 complexes. The presence of Li+ ions decreased the curing temperature significantly to 186 °C, suggesting that the metal ions functioned as an effective catalyst and promoter that accelerated the ring opening polymerization of the crown-ether BZ monomer. The ionic conductivity reached 8.3 × 10–5 S cm–1 for the crown-ether BZ/LiClO4 = 90/10 complex after thermal curing; this value is higher than those of typical polymer-based systems (e.g., PEO, PCL, PMMA, and PVP) while also providing a polymer electrolyte of higher thermal stability.
中文翻译:

冠醚功能化聚苯并恶嗪用于金属离子吸附
在这项研究中,我们通过还原由二苯并[18]冠-6二胺衍生物和水杨醛制得的席夫碱,并随后将其反应,以高收率和纯度合成了一种新的冠醚官能化苯并恶嗪单体(冠醚BZ)。所得ø用CH -hydroxybenzylamine物种2 O.我们使用差示扫描量热法(DSC),傅里叶变换红外光谱(FTIR),和热重分析来检查期间各种类型的热开环聚合和冠醚BZ单体的热稳定性热处理。DSC显示,该冠醚BZ单体具有较低的固化温度(210°C;典型的Pa型3-苯基-3,4-二氢-2 H)-苯并恶嗪单体:263°C),因为主链主链结构上冠醚部分的柔韧性催化了开环聚合。我们还使用DSC,FTIR光谱和离子电导率测量来研究冠醚BZ / LiClO 4配合物的特定金属-冠醚相互作用。Li +离子的存在将固化温度显着降低至186°C,这表明金属离子可作为有效的催化剂和促进剂,促进冠醚BZ单体的开环聚合。冠醚BZ / LiClO 4的离子电导率达到8.3×10 –5 S cm –1=热固化后为90/10的络合物;该值高于典型的基于聚合物的系统(例如,PEO,PCL,PMMA和PVP),同时还提供了具有更高热稳定性的聚合物电解质。
更新日期:2020-04-24
中文翻译:

冠醚功能化聚苯并恶嗪用于金属离子吸附
在这项研究中,我们通过还原由二苯并[18]冠-6二胺衍生物和水杨醛制得的席夫碱,并随后将其反应,以高收率和纯度合成了一种新的冠醚官能化苯并恶嗪单体(冠醚BZ)。所得ø用CH -hydroxybenzylamine物种2 O.我们使用差示扫描量热法(DSC),傅里叶变换红外光谱(FTIR),和热重分析来检查期间各种类型的热开环聚合和冠醚BZ单体的热稳定性热处理。DSC显示,该冠醚BZ单体具有较低的固化温度(210°C;典型的Pa型3-苯基-3,4-二氢-2 H)-苯并恶嗪单体:263°C),因为主链主链结构上冠醚部分的柔韧性催化了开环聚合。我们还使用DSC,FTIR光谱和离子电导率测量来研究冠醚BZ / LiClO 4配合物的特定金属-冠醚相互作用。Li +离子的存在将固化温度显着降低至186°C,这表明金属离子可作为有效的催化剂和促进剂,促进冠醚BZ单体的开环聚合。冠醚BZ / LiClO 4的离子电导率达到8.3×10 –5 S cm –1=热固化后为90/10的络合物;该值高于典型的基于聚合物的系统(例如,PEO,PCL,PMMA和PVP),同时还提供了具有更高热稳定性的聚合物电解质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号