当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and leishmanicidal evaluation of sulfanyl‐ and sulfonyl‐tethered functionalized benzoate derivatives featuring a nitroimidazole moiety
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-03-16 , DOI: 10.1002/ardp.202000002 Miguel Rodríguez 1 , Joyce Gutiérrez 1 , José Domínguez 1 , Philippe A Peixoto 2 , Alexis Fernández 3 , Noris Rodríguez 3 , Denis Deffieux 2 , Luis Rojas 4 , Stéphane Quideau 2 , Laurent Pouységu 2 , Jaime Charris 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-03-16 , DOI: 10.1002/ardp.202000002 Miguel Rodríguez 1 , Joyce Gutiérrez 1 , José Domínguez 1 , Philippe A Peixoto 2 , Alexis Fernández 3 , Noris Rodríguez 3 , Denis Deffieux 2 , Luis Rojas 4 , Stéphane Quideau 2 , Laurent Pouységu 2 , Jaime Charris 1
Affiliation
|
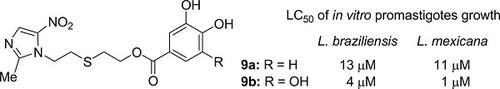
A series of new nitroimidazole‐containing derivatives was synthesized by coupling of 2‐[2‐(2‐methyl‐5‐nitro‐1H‐imidazol‐1‐yl)ethylthio]ethanol with diversely substituted benzoic acids. Upon treatment with m‐CPBA, 12 of these sulfanyl compounds were further oxidized to their sulfonyl analogs. All the 26 synthetic compounds were examined for in vitro activity against Leishmania (V.) braziliensis and Leishmania (L.) mexicana, and some of them displayed an efficient antileishmanial activity. Among the compounds tested, the catecholic derivative 2‐{[2‐(2‐methyl‐5‐nitro‐1H‐imidazol‐1‐yl)ethyl]sulfanyl}ethyl 3,4‐dihydroxybenzoate (9a, LC50 = 13 and 11 µM) and the pyrogallolic derivative 2‐{[2‐(2‐methyl‐5‐nitro‐1H‐imidazol‐1‐yl)ethyl]sulfanyl}ethyl 3,4,5‐trihydroxybenzoate (9b, LC50 = 4 and 1 µM) were the most active ones against the two Leishmania strains.
中文翻译:

具有硝基咪唑部分的磺酰基和磺酰基连接的官能化苯甲酸酯衍生物的合成和利什曼原虫杀灭评估
通过将 2-[2-(2-甲基-5-硝基-1H-咪唑-1-基)乙硫基]乙醇与不同取代的苯甲酸偶联,合成了一系列新的含硝基咪唑的衍生物。用 m-CPBA 处理后,这些硫酰基化合物中有 12 种被进一步氧化成它们的磺酰基类似物。检查了所有 26 种合成化合物对巴西利什曼原虫 (V.) 和墨西哥利什曼原虫 (L.) 的体外活性,其中一些显示出有效的抗利什曼原虫活性。在测试的化合物中,儿茶酚衍生物 2-{[2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl]sulfanyl}ethyl 3,4-dihydroxybenzoate (9a, LC50 = 13 and 11 µM ) 和邻苯三酚衍生物 2-{[2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl]sulfanyl}3,4,5-trihydroxybenzoate (9b,
更新日期:2020-03-16
中文翻译:

具有硝基咪唑部分的磺酰基和磺酰基连接的官能化苯甲酸酯衍生物的合成和利什曼原虫杀灭评估
通过将 2-[2-(2-甲基-5-硝基-1H-咪唑-1-基)乙硫基]乙醇与不同取代的苯甲酸偶联,合成了一系列新的含硝基咪唑的衍生物。用 m-CPBA 处理后,这些硫酰基化合物中有 12 种被进一步氧化成它们的磺酰基类似物。检查了所有 26 种合成化合物对巴西利什曼原虫 (V.) 和墨西哥利什曼原虫 (L.) 的体外活性,其中一些显示出有效的抗利什曼原虫活性。在测试的化合物中,儿茶酚衍生物 2-{[2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl]sulfanyl}ethyl 3,4-dihydroxybenzoate (9a, LC50 = 13 and 11 µM ) 和邻苯三酚衍生物 2-{[2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl]sulfanyl}3,4,5-trihydroxybenzoate (9b,











































 京公网安备 11010802027423号
京公网安备 11010802027423号