当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Efficient Chemo‐Selective N‐Alkylation Methodology for the Structure Diversification of Indolylbenzimidazoles
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-17 , DOI: 10.1002/slct.201904745 Leonard Barasa 1 , Alison Yong 1 , Sabesan Yoganathan 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-17 , DOI: 10.1002/slct.201904745 Leonard Barasa 1 , Alison Yong 1 , Sabesan Yoganathan 1
Affiliation
|
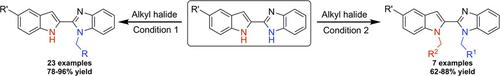
Indolylbenzimidazoles have emerged as an important pharmacophore during drug discovery efforts. More specifically, N‐alkylation of indole and/or benzimidazole motifs has been a useful strategy for structure diversification to generate bioactive leads. Herein, we report a simple and efficient methodology for the chemo‐selective N‐alkylation of indolylbenzimidazole scaffolds. This approach takes advantage of the pKa differences between the indole and benzimidazole nitrogen to achieve the desired chemo‐selectivity. Using the reported method, one can readily access a selection of mono‐N‐alkylated or asymmetrically bis‐alkylated indolylbenzimidazole scaffolds in a simple one‐pot operation. Moreover, this method provides the desired products in excellent yield and demonstrates a broad substrate scope.
中文翻译:

吲哚基苯并咪唑类结构多样化的高效化学选择性N烷基化方法
在药物开发过程中,吲哚基苯并咪唑已成为一种重要的药效团。更具体地说,吲哚和/或苯并咪唑基序的N-烷基化已成为结构多样化以产生生物活性先导的有用策略。在这里,我们报告了一种简单有效的方法,用于吲哚基苯并咪唑支架的化学选择性N烷基化。这种方法利用了吲哚和苯并咪唑氮之间的pKa差异来实现所需的化学选择性。利用报道的方法,可以容易地访问一个选择单Ñ简单的一锅操作即可完成烷基化或不对称双烷基化的吲哚基苯并咪唑支架。此外,该方法以优异的产率提供了所需的产物,并证明了广泛的底物范围。
更新日期:2020-03-19
中文翻译:

吲哚基苯并咪唑类结构多样化的高效化学选择性N烷基化方法
在药物开发过程中,吲哚基苯并咪唑已成为一种重要的药效团。更具体地说,吲哚和/或苯并咪唑基序的N-烷基化已成为结构多样化以产生生物活性先导的有用策略。在这里,我们报告了一种简单有效的方法,用于吲哚基苯并咪唑支架的化学选择性N烷基化。这种方法利用了吲哚和苯并咪唑氮之间的pKa差异来实现所需的化学选择性。利用报道的方法,可以容易地访问一个选择单Ñ简单的一锅操作即可完成烷基化或不对称双烷基化的吲哚基苯并咪唑支架。此外,该方法以优异的产率提供了所需的产物,并证明了广泛的底物范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号