Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular mechanisms of K+ clearance and extracellular space shrinkage-Glia cells as the stars.
Glia ( IF 5.4 ) Pub Date : 2020-03-17 , DOI: 10.1002/glia.23824 Nanna MacAulay 1
Glia ( IF 5.4 ) Pub Date : 2020-03-17 , DOI: 10.1002/glia.23824 Nanna MacAulay 1
Affiliation
|
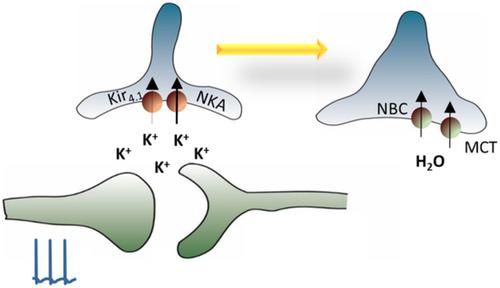
Neuronal signaling in the central nervous system (CNS) associates with release of K+ into the extracellular space resulting in transient increases in [K+]o. This elevated K+ is swiftly removed, in part, via uptake by neighboring glia cells. This process occurs in parallel to the [K+]o elevation and glia cells thus act as K+ sinks during the neuronal activity, while releasing it at the termination of the pulse. The molecular transport mechanisms governing this glial K+ absorption remain a point of debate. Passive distribution of K+ via Kir4.1‐mediated spatial buffering of K+ has become a favorite within the glial field, although evidence for a quantitatively significant contribution from this ion channel to K+ clearance from the extracellular space is sparse. The Na+/K+‐ATPase, but not the Na+/K+/Cl− cotransporter, NKCC1, shapes the activity‐evoked K+ transient. The different isoform combinations of the Na+/K+‐ATPase expressed in glia cells and neurons display different kinetic characteristics and are thereby distinctly geared toward their temporal and quantitative contribution to K+ clearance. The glia cell swelling occurring with the K+ transient was long assumed to be directly associated with K+ uptake and/or AQP4, although accumulating evidence suggests that they are not. Rather, activation of bicarbonate‐ and lactate transporters appear to lead to glial cell swelling via the activity‐evoked alkaline transient, K+‐mediated glial depolarization, and metabolic demand. This review covers evidence, or lack thereof, accumulated over the last half century on the molecular mechanisms supporting activity‐evoked K+ and extracellular space dynamics.
中文翻译:

K+清除和细胞外空间收缩的分子机制——神经胶质细胞作为恒星。
中枢神经系统 (CNS) 中的神经元信号与 K +释放到细胞外空间相关,导致 [K + ] o 的瞬时增加。这种升高的 K +被迅速去除,部分是通过相邻神经胶质细胞的摄取。这个过程与[K + ] o升高并行发生,因此神经胶质细胞在神经元活动期间充当K +汇,而在脉冲终止时释放它。控制这种胶质 K +吸收的分子传输机制仍然是一个争论的焦点。K +通过 Kir4.1 介导的 K +空间缓冲被动分布已成为神经胶质领域的最爱,尽管这种离子通道对从细胞外空间清除K +的定量显着贡献的证据很少。Na + /K + ‐ATPase,而不是 Na + /K + /Cl -协同转运蛋白 NKCC1,塑造了活性诱发的 K +瞬态。在神经胶质细胞和神经元中表达的 Na + /K + -ATPase的不同同工型组合显示出不同的动力学特征,因此明显地针对它们对 K + 的时间和数量贡献清除。长期以来,人们一直认为K +瞬变引起的神经胶质细胞肿胀与 K +摄取和/或 AQP4直接相关,尽管越来越多的证据表明它们并非如此。相反,碳酸氢盐和乳酸转运蛋白的激活似乎通过活性诱发的碱性瞬变、K +介导的神经胶质去极化和代谢需求导致神经胶质细胞肿胀。这篇综述涵盖了过去半个世纪积累的关于支持活动诱发的 K +和细胞外空间动力学的分子机制的证据或缺乏证据。
更新日期:2020-03-17
中文翻译:

K+清除和细胞外空间收缩的分子机制——神经胶质细胞作为恒星。
中枢神经系统 (CNS) 中的神经元信号与 K +释放到细胞外空间相关,导致 [K + ] o 的瞬时增加。这种升高的 K +被迅速去除,部分是通过相邻神经胶质细胞的摄取。这个过程与[K + ] o升高并行发生,因此神经胶质细胞在神经元活动期间充当K +汇,而在脉冲终止时释放它。控制这种胶质 K +吸收的分子传输机制仍然是一个争论的焦点。K +通过 Kir4.1 介导的 K +空间缓冲被动分布已成为神经胶质领域的最爱,尽管这种离子通道对从细胞外空间清除K +的定量显着贡献的证据很少。Na + /K + ‐ATPase,而不是 Na + /K + /Cl -协同转运蛋白 NKCC1,塑造了活性诱发的 K +瞬态。在神经胶质细胞和神经元中表达的 Na + /K + -ATPase的不同同工型组合显示出不同的动力学特征,因此明显地针对它们对 K + 的时间和数量贡献清除。长期以来,人们一直认为K +瞬变引起的神经胶质细胞肿胀与 K +摄取和/或 AQP4直接相关,尽管越来越多的证据表明它们并非如此。相反,碳酸氢盐和乳酸转运蛋白的激活似乎通过活性诱发的碱性瞬变、K +介导的神经胶质去极化和代谢需求导致神经胶质细胞肿胀。这篇综述涵盖了过去半个世纪积累的关于支持活动诱发的 K +和细胞外空间动力学的分子机制的证据或缺乏证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号