当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Copper‐Catalyzed Radical Ring‐Opening Cyanation of Cyclopropanols and Cyclopropanone Acetals
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-16 , DOI: 10.1002/adsc.202000202 Lianqian Wu 1 , Lei Wang 1 , Pinghong Chen 1 , Yin‐Long Guo 1 , Guosheng Liu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-16 , DOI: 10.1002/adsc.202000202 Lianqian Wu 1 , Lei Wang 1 , Pinghong Chen 1 , Yin‐Long Guo 1 , Guosheng Liu 1
Affiliation
|
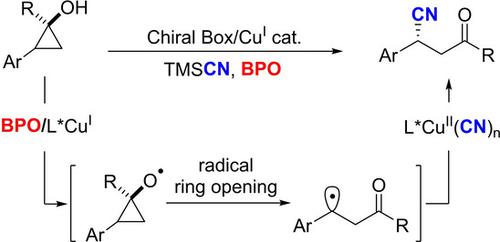
A novel approach for enantioselective cyanation of cyclopropanols and their derivatives through copper‐catalyzed radical relay processes has been developed. Various cyclopropanols and cyclopropanone acetals are compatible to the catalytic conditions, providing β‐carbonyl nitriles with excellent enantioselectivity. These products can be readily converted to chiral γ‐amino acids derivatives and drugs such as (R)‐baclofen. Preliminary mechanistic studies have supported a ring‐opening process for cyclopropanoxy radicals followed by copper‐catalyzed enantioselective cyanation of benzylic radicals to form the C−CN bonds in an enantioselective manner.
中文翻译:

对映选择性铜催化环丙醇和环丙酮酮缩醛的自由基开环氰化
通过铜催化的自由基中继过程,开发了一种新的环丙醇及其衍生物的对映选择性氰化方法。各种环丙醇和环丙酮缩醛均与催化条件兼容,从而为β-羰基腈提供了出色的对映选择性。这些产品可以很容易地转化为手性γ-氨基酸衍生物和药物,例如(R)-baclofen。初步的机理研究支持了环丙氧基自由基的开环过程,随后是铜催化的苄基自由基的对映选择性氰化,以对映选择性的方式形成C-CN键。
更新日期:2020-03-16
中文翻译:

对映选择性铜催化环丙醇和环丙酮酮缩醛的自由基开环氰化
通过铜催化的自由基中继过程,开发了一种新的环丙醇及其衍生物的对映选择性氰化方法。各种环丙醇和环丙酮缩醛均与催化条件兼容,从而为β-羰基腈提供了出色的对映选择性。这些产品可以很容易地转化为手性γ-氨基酸衍生物和药物,例如(R)-baclofen。初步的机理研究支持了环丙氧基自由基的开环过程,随后是铜催化的苄基自由基的对映选择性氰化,以对映选择性的方式形成C-CN键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号