当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate Recognition and Catalytic Mechanism of the Phosphate Acyltransferase PlsX from Bacillus subtilis.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-03-16 , DOI: 10.1002/cbic.202000015 Yiping Jiang 1 , Mingming Qin 1 , Zhihong Guo 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-03-16 , DOI: 10.1002/cbic.202000015 Yiping Jiang 1 , Mingming Qin 1 , Zhihong Guo 1
Affiliation
|
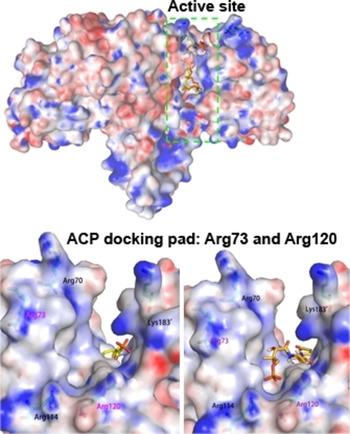
Doing different : The peripheral phosphate acyltransferase PlsX is shown to have an active‐site architecture different from that of phosphotransacetylases and to catalyse a similar acyl transfer reaction through a different mechanism. The catalysis probably involves substrate activation and transition‐state stabilization by two conserved residues, a lysine and an asparagine.

中文翻译:

枯草芽孢杆菌磷酸酰基转移酶PlsX的底物识别和催化机理。
做不同的:将周边磷酸酰基转移酶PLSX被示出为具有与phosphotransacetylases的活性位点的体系结构不同,并且以催化通过不同的机制类似的酰基转移反应。催化作用可能涉及底物活化和两个保守残基赖氨酸和天冬酰胺的过渡态稳定化。

更新日期:2020-03-16

中文翻译:

枯草芽孢杆菌磷酸酰基转移酶PlsX的底物识别和催化机理。
做不同的:将周边磷酸酰基转移酶PLSX被示出为具有与phosphotransacetylases的活性位点的体系结构不同,并且以催化通过不同的机制类似的酰基转移反应。催化作用可能涉及底物活化和两个保守残基赖氨酸和天冬酰胺的过渡态稳定化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号