当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ru-doped, oxygen-vacancy-containing CeO2 nanorods toward N2 electroreduction
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020/03/18 , DOI: 10.1039/d0ta02211j Yu Ding 1, 2, 3, 4, 5 , Linsong Huang 1, 2, 3, 4, 5 , Junbo Zhang 1, 2, 3, 4, 5 , Anxiang Guan 1, 2, 3, 4, 5 , Qihao Wang 1, 2, 3, 4, 5 , Linping Qian 1, 2, 3, 4, 5 , Lijuan Zhang 1, 2, 3, 4, 5 , Gengfeng Zheng 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020/03/18 , DOI: 10.1039/d0ta02211j Yu Ding 1, 2, 3, 4, 5 , Linsong Huang 1, 2, 3, 4, 5 , Junbo Zhang 1, 2, 3, 4, 5 , Anxiang Guan 1, 2, 3, 4, 5 , Qihao Wang 1, 2, 3, 4, 5 , Linping Qian 1, 2, 3, 4, 5 , Lijuan Zhang 1, 2, 3, 4, 5 , Gengfeng Zheng 1, 2, 3, 4, 5
Affiliation
|
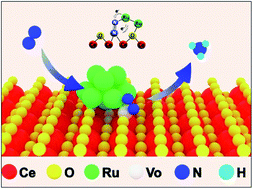
Electrochemical N2 reduction (N2RR) in aqueous solutions under ambient conditions is extremely challenging; thus, a rational design of electrocatalytic centers is required to effectively adsorb and activate N2. Herein, we report Ru cluster-doped, oxygen vacancy-rich CeO2 (Ru/CeO2-VO) nanorods as an efficient N2 reduction electrocatalyst. The high-density oxygen vacancies inside CeO2 are beneficial for the adsorption of N2, and the unsaturated, low-valence Ru nanoclusters provide active sites for N2 activation and subsequent electron transfer for N2 reduction. The Ru/CeO2-VO catalyst exhibits a high ammonia production rate of 9.87 × 10−8 mmol s−1 cm−2 and a partial current density of 32 μA cm−2 at −0.25 V versus reversible hydrogen electrode in 0.05 M H2SO4 solution, corresponding to a faradaic efficiency of 11.7%. Our work suggests that the synergistic effects between Ru nanoclusters and oxygen vacancies in CeO2 for efficient N2 fixation.
中文翻译:

掺钌,含氧空位的CeO2纳米棒对N2电还原的影响
在环境条件下水溶液中的电化学N 2还原(N 2 RR)极具挑战性。因此,需要合理设计电催化中心以有效地吸附和活化N 2。在本文中,我们报道孺簇掺杂的,富氧空位的CeO 2(RU /的CeO 2 -V ö)纳米棒作为一种高效ñ 2还原电催化剂。CeO 2内的高密度氧空位有利于N 2的吸附,不饱和的低价Ru纳米簇为N 2活化和随后的N 2电子转移提供了活性位点减少。与0.05 MH中的可逆氢电极相比,Ru / CeO 2 -V O催化剂在-0.25 V时表现出9.87×10 -8 mmol s -1 cm -2的高氨生成率和32μAcm -2的分流密度2 SO 4溶液,对应的法拉第效率为11.7%。我们的工作表明,Ru纳米团簇与CeO 2中的氧空位之间的协同效应可有效固定N 2。
更新日期:2020-04-15
中文翻译:

掺钌,含氧空位的CeO2纳米棒对N2电还原的影响
在环境条件下水溶液中的电化学N 2还原(N 2 RR)极具挑战性。因此,需要合理设计电催化中心以有效地吸附和活化N 2。在本文中,我们报道孺簇掺杂的,富氧空位的CeO 2(RU /的CeO 2 -V ö)纳米棒作为一种高效ñ 2还原电催化剂。CeO 2内的高密度氧空位有利于N 2的吸附,不饱和的低价Ru纳米簇为N 2活化和随后的N 2电子转移提供了活性位点减少。与0.05 MH中的可逆氢电极相比,Ru / CeO 2 -V O催化剂在-0.25 V时表现出9.87×10 -8 mmol s -1 cm -2的高氨生成率和32μAcm -2的分流密度2 SO 4溶液,对应的法拉第效率为11.7%。我们的工作表明,Ru纳米团簇与CeO 2中的氧空位之间的协同效应可有效固定N 2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号