当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the failure mechanism of transition-metal chalcogenides towards the copper current collector in secondary batteries
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020/03/17 , DOI: 10.1039/d0ta00622j Guannan Zu 1, 2, 3, 4, 5 , Gencai Guo 1, 2, 3, 4, 5 , Hongyi Li 1, 2, 3, 4, 5 , Yue Lu 3, 4, 5, 6 , Ruzhi Wang 1, 2, 3, 4, 5 , Yuxiang Hu 7, 8, 9, 10, 11 , Lianzhou Wang 7, 8, 9, 10, 11 , Jinshu Wang 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020/03/17 , DOI: 10.1039/d0ta00622j Guannan Zu 1, 2, 3, 4, 5 , Gencai Guo 1, 2, 3, 4, 5 , Hongyi Li 1, 2, 3, 4, 5 , Yue Lu 3, 4, 5, 6 , Ruzhi Wang 1, 2, 3, 4, 5 , Yuxiang Hu 7, 8, 9, 10, 11 , Lianzhou Wang 7, 8, 9, 10, 11 , Jinshu Wang 1, 2, 3, 4, 5
Affiliation
|
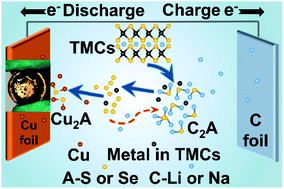
Transition-metal chalcogenides (TMCs) are attractive high-capacity anode candidates for secondary batteries, but they suffer from significant challenges including dramatic volume expansion and rapid capacity fading. Despite considerable progress, it was still a big challenge to achieve hundreds of stable cycles with high capacity retention. Herein, we reveal that the copper (Cu) current collector corrosion due to the sulphur (S) species attack leads to serious decay in anode performance in TMC-based batteries, as evidenced by the side-reaction product Cu2S generated via the consumption of S and Cu on the cycled TMC electrode. The electrochemical test and DFT calculations both reveal that the resultant Cu2S increases the resistance at interfaces and reduces the charge transfer, leading to a low capacity and short lifespan. By simply introducing a conductive layer between Cu foil and TMCs, the corrosive influence of Cu is remarkably suppressed, resulting in much enhanced cycling performance. At current densities of 0.5C and 1C, the Cu/2hTi/MoS2 sample delivered a high discharge capacity of 1010 and 740 mA h g−1 after 400 cycles, respectively. This work provides new insights to understand the fundamental mechanism of the electrochemical reactions and strategy to improve the energy storage ability of TMCs.
中文翻译:

揭示过渡金属硫属元素化物对二次电池中铜集电器的破坏机理
过渡金属硫族化物(TMC)是用于二次电池的有吸引力的高容量阳极候选材料,但它们面临着巨大的挑战,包括急剧的体积膨胀和快速的容量衰减。尽管取得了长足的进步,但要实现数百个稳定周期并保持高容量仍是一个巨大的挑战。本文中,我们揭示了由于硫(S)物种腐蚀而导致的铜(Cu)集电器腐蚀导致TMC基电池的阳极性能严重下降,这通过消耗产生的副反应产物Cu 2 S得以证明。S和Cu在循环TMC电极上的含量。电化学测试和DFT计算均表明生成的Cu 2S增加了界面处的电阻并减少了电荷转移,导致容量低和寿命短。通过简单地在铜箔和TMC之间引入导电层,可显着抑制铜的腐蚀影响,从而大大提高循环性能。在电流密度为0.5C和1C时,Cu / 2hTi / MoS 2样品在400个循环后分别具有1010和740 mA hg -1的高放电容量。这项工作提供了新的见解,以了解电化学反应的基本机理和提高TMC储能能力的策略。
更新日期:2020-04-08
中文翻译:

揭示过渡金属硫属元素化物对二次电池中铜集电器的破坏机理
过渡金属硫族化物(TMC)是用于二次电池的有吸引力的高容量阳极候选材料,但它们面临着巨大的挑战,包括急剧的体积膨胀和快速的容量衰减。尽管取得了长足的进步,但要实现数百个稳定周期并保持高容量仍是一个巨大的挑战。本文中,我们揭示了由于硫(S)物种腐蚀而导致的铜(Cu)集电器腐蚀导致TMC基电池的阳极性能严重下降,这通过消耗产生的副反应产物Cu 2 S得以证明。S和Cu在循环TMC电极上的含量。电化学测试和DFT计算均表明生成的Cu 2S增加了界面处的电阻并减少了电荷转移,导致容量低和寿命短。通过简单地在铜箔和TMC之间引入导电层,可显着抑制铜的腐蚀影响,从而大大提高循环性能。在电流密度为0.5C和1C时,Cu / 2hTi / MoS 2样品在400个循环后分别具有1010和740 mA hg -1的高放电容量。这项工作提供了新的见解,以了解电化学反应的基本机理和提高TMC储能能力的策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号