当前位置:
X-MOL 学术
›
PLOS Genet.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A homozygous missense variant in CACNB4 encoding the auxiliary calcium channel beta4 subunit causes a severe neurodevelopmental disorder and impairs channel and non-channel functions.
PLOS Genetics ( IF 4.0 ) Pub Date : 2020-03-16 , DOI: 10.1371/journal.pgen.1008625 Pierre Coste de Bagneaux 1 , Leonie von Elsner 2 , Tatjana Bierhals 2 , Marta Campiglio 1 , Jessika Johannsen 3 , Gerald J Obermair 1, 4 , Maja Hempel 2 , Bernhard E Flucher 1 , Kerstin Kutsche 2
PLOS Genetics ( IF 4.0 ) Pub Date : 2020-03-16 , DOI: 10.1371/journal.pgen.1008625 Pierre Coste de Bagneaux 1 , Leonie von Elsner 2 , Tatjana Bierhals 2 , Marta Campiglio 1 , Jessika Johannsen 3 , Gerald J Obermair 1, 4 , Maja Hempel 2 , Bernhard E Flucher 1 , Kerstin Kutsche 2
Affiliation
|
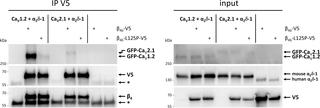
P/Q-type channels are the principal presynaptic calcium channels in brain functioning in neurotransmitter release. They are composed of the pore-forming CaV2.1 α1 subunit and the auxiliary α2δ-2 and β4 subunits. β4 is encoded by CACNB4, and its multiple splice variants serve isoform-specific functions as channel subunits and transcriptional regulators in the nucleus. In two siblings with intellectual disability, psychomotor retardation, blindness, epilepsy, movement disorder and cerebellar atrophy we identified rare homozygous variants in the genes LTBP1, EMILIN1, CACNB4, MINAR1, DHX38 and MYO15 by whole-exome sequencing. In silico tools, animal model, clinical, and genetic data suggest the p.(Leu126Pro) CACNB4 variant to be likely pathogenic. To investigate the functional consequences of the CACNB4 variant, we introduced the corresponding mutation L125P into rat β4b cDNA. Heterologously expressed wild-type β4b associated with GFP-CaV1.2 and accumulated in presynaptic boutons of cultured hippocampal neurons. In contrast, the β4b-L125P mutant failed to incorporate into calcium channel complexes and to cluster presynaptically. When co-expressed with CaV2.1 in tsA201 cells, β4b and β4b-L125P augmented the calcium current amplitudes, however, β4b-L125P failed to stably complex with α1 subunits. These results indicate that p.Leu125Pro disrupts the stable association of β4b with native calcium channel complexes, whereas membrane incorporation, modulation of current density and activation properties of heterologously expressed channels remained intact. Wildtype β4b was specifically targeted to the nuclei of quiescent excitatory cells. Importantly, the p.Leu125Pro mutation abolished nuclear targeting of β4b in cultured myotubes and hippocampal neurons. While binding of β4b to the known interaction partner PPP2R5D (B56δ) was not affected by the mutation, complex formation between β4b-L125P and the neuronal TRAF2 and NCK interacting kinase (TNIK) seemed to be disturbed. In summary, our data suggest that the homozygous CACNB4 p.(Leu126Pro) variant underlies the severe neurological phenotype in the two siblings, most likely by impairing both channel and non-channel functions of β4b.
中文翻译:

CACNB4 中编码辅助钙通道 β4 亚基的纯合错义变异会导致严重的神经发育障碍并损害通道和非通道功能。
P/Q 型通道是大脑中神经递质释放功能的主要突触前钙通道。它们由成孔 CaV2.1 α1 亚基和辅助 α2δ-2 和 β4 亚基组成。β4 由 CACNB4 编码,其多个剪接变体作为细胞核中的通道亚基和转录调节因子发挥异构体特异性功能。在患有智力障碍、精神运动迟缓、失明、癫痫、运动障碍和小脑萎缩的两个兄弟姐妹中,我们通过全外显子组测序在基因 LTBP1、EMILIN1、CACNB4、MINAR1、DHX38 和 MYO15 中发现了罕见的纯合变异。在计算机模拟工具中,动物模型、临床和遗传数据表明 p.(Leu126Pro) CACNB4 变体可能具有致病性。为了研究 CACNB4 变体的功能后果,我们将相应的突变 L125P 引入大鼠 β4b cDNA 中。异源表达的野生型 β4b 与 GFP-CaV1.2 相关,并在培养的海马神经元的突触前束中积累。相反,β4b-L125P 突变体未能融入钙通道复合物并在突触前聚集。当在 tsA201 细胞中与 CaV2.1 共表达时,β4b 和 β4b-L125P 增强了钙电流幅度,然而,β4b-L125P 未能与 α1 亚基稳定复合。这些结果表明,p.Leu125Pro 破坏了 β4b 与天然钙通道复合物的稳定结合,而异源表达通道的膜掺入、电流密度调节和激活特性保持完整。野生型 β4b 专门针对静止兴奋细胞的细胞核。重要的是,p.Leu125Pro 突变消除了培养的肌管和海马神经元中 β4b 的核靶向。虽然 β4b 与已知相互作用伙伴 PPP2R5D (B56δ) 的结合不受突变影响,但 β4b-L125P 与神经元 TRAF2 和 NCK 相互作用激酶 (TNIK) 之间的复合物形成似乎受到干扰。总之,我们的数据表明,纯合的 CACNB4 p.(Leu126Pro) 变异是这两个兄弟姐妹严重神经表型的基础,很可能是通过损害 β4b 的通道和非通道功能来实现的。
更新日期:2020-04-06
中文翻译:

CACNB4 中编码辅助钙通道 β4 亚基的纯合错义变异会导致严重的神经发育障碍并损害通道和非通道功能。
P/Q 型通道是大脑中神经递质释放功能的主要突触前钙通道。它们由成孔 CaV2.1 α1 亚基和辅助 α2δ-2 和 β4 亚基组成。β4 由 CACNB4 编码,其多个剪接变体作为细胞核中的通道亚基和转录调节因子发挥异构体特异性功能。在患有智力障碍、精神运动迟缓、失明、癫痫、运动障碍和小脑萎缩的两个兄弟姐妹中,我们通过全外显子组测序在基因 LTBP1、EMILIN1、CACNB4、MINAR1、DHX38 和 MYO15 中发现了罕见的纯合变异。在计算机模拟工具中,动物模型、临床和遗传数据表明 p.(Leu126Pro) CACNB4 变体可能具有致病性。为了研究 CACNB4 变体的功能后果,我们将相应的突变 L125P 引入大鼠 β4b cDNA 中。异源表达的野生型 β4b 与 GFP-CaV1.2 相关,并在培养的海马神经元的突触前束中积累。相反,β4b-L125P 突变体未能融入钙通道复合物并在突触前聚集。当在 tsA201 细胞中与 CaV2.1 共表达时,β4b 和 β4b-L125P 增强了钙电流幅度,然而,β4b-L125P 未能与 α1 亚基稳定复合。这些结果表明,p.Leu125Pro 破坏了 β4b 与天然钙通道复合物的稳定结合,而异源表达通道的膜掺入、电流密度调节和激活特性保持完整。野生型 β4b 专门针对静止兴奋细胞的细胞核。重要的是,p.Leu125Pro 突变消除了培养的肌管和海马神经元中 β4b 的核靶向。虽然 β4b 与已知相互作用伙伴 PPP2R5D (B56δ) 的结合不受突变影响,但 β4b-L125P 与神经元 TRAF2 和 NCK 相互作用激酶 (TNIK) 之间的复合物形成似乎受到干扰。总之,我们的数据表明,纯合的 CACNB4 p.(Leu126Pro) 变异是这两个兄弟姐妹严重神经表型的基础,很可能是通过损害 β4b 的通道和非通道功能来实现的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号