PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-03-16 , DOI: 10.1371/journal.ppat.1008447 Yuyan Wang 1 , Shujuan Du 1 , Caixia Zhu 1 , Chong Wang 1 , Nuoya Yu 1 , Ziqi Lin 1 , Jin Gan 1, 2 , Yi Guo 3 , Xinxin Huang 4 , Yuping He 4 , Erle Robertson 5 , Di Qu 1 , Fang Wei 2 , Qiliang Cai 1, 6
|
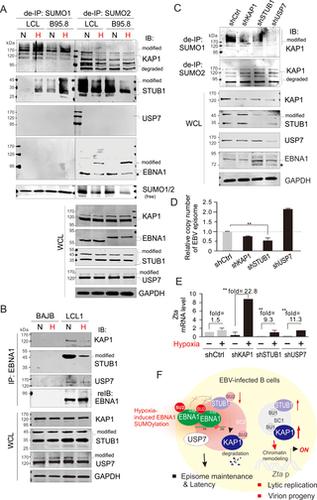
Latent Epstein-Barr virus (EBV) infection is strongly associated with several malignancies, including B-cell lymphomas and epithelial tumors. EBNA1 is a key antigen expressed in all EBV-associated tumors during latency that is required for maintenance of the EBV episome DNA and the regulation of viral gene transcription. However, the mechanism utilized by EBV to maintain latent infection at the levels of posttranslational regulation remains largely unclear. Here, we report that EBNA1 contains two SUMO-interacting motifs (SIM2 and SIM3), and mutation of SIM2, but not SIM3, dramatically disrupts the EBNA1 dimerization, while SIM3 contributes to the polySUMO2 modification of EBNA1 at lysine 477 in vitro. Proteomic and immunoprecipitation analyses further reveal that the SIM3 motif is required for the EBNA1-mediated inhibitory effects on SUMO2-modified STUB1, SUMO2-mediated degradation of USP7, and SUMO1-modified KAP1. Deletion of the EBNASIM motif leads to functional loss of both EBNA1-mediated viral episome maintenance and lytic gene silencing. Importantly, hypoxic stress induces the SUMO2 modification of EBNA1, and in turn the dissociation of EBNA1 with STUB1, KAP1 and USP7 to increase the SUMO1 modification of both STUB1 and KAP1 for reactivation of lytic replication. Therefore, the EBNA1SIM motif plays an essential role in EBV latency and is a potential therapeutic target against EBV-associated cancers.
中文翻译:

STUB1被EBNA1的SUMO相互作用基序靶向,以维持爱泼斯坦-巴尔病毒潜伏期。
潜在的爱泼斯坦-巴尔病毒(EBV)感染与多种恶性肿瘤密切相关,包括B细胞淋巴瘤和上皮肿瘤。EBNA1是在维持潜伏期病毒附加体DNA和调节病毒基因转录所需的潜伏期中在所有与EBV相关的肿瘤中表达的关键抗原。但是,EBV用于在翻译后调节水平上保持潜伏感染的机制仍然不清楚。在这里,我们报道EBNA1包含两个SUMO交互基元(SIM2和SIM3),而SIM2而不是SIM3的突变会极大地破坏EBNA1的二聚化,而SIM3在体外对EBNA1的polySUMO2赖氨酸477修饰有贡献。蛋白质组学和免疫沉淀分析进一步表明,SIM3基序是EBNA1介导的对SUMO2修饰的STUB1,SUMO2介导的USP7降解和SUMO1修饰的KAP1抑制作用所必需的。EBNA SIM基序的删除会导致EBNA1介导的病毒附加体维持功能和裂解基因沉默功能丧失。重要的是,低氧胁迫诱导EBNA1的SUMO2修饰,进而使EBNA1与STUB1,KAP1和USP7分离,从而增加STUB1和KAP1的SUMO1修饰,以重新激活裂解复制。因此,EBNA1 SIM基序在EBV潜伏期中起重要作用,并且是针对EBV相关癌症的潜在治疗靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号