PLOS Medicine ( IF 10.5 ) Pub Date : 2020-03-17 , DOI: 10.1371/journal.pmed.1003024 Angus H Forster 1 , Katey Witham 1 , Alexandra C I Depelsenaire 1 , Margaret Veitch 2 , James W Wells 2 , Adam Wheatley 3 , Melinda Pryor 4 , Jason D Lickliter 5 , Barbara Francis 6 , Steve Rockman 3, 7 , Jesse Bodle 7 , Peter Treasure 8 , Julian Hickling 9 , Germain J P Fernando 1, 10
|
Background
The Vaxxas high-density microarray patch (HD-MAP) consists of a high density of microprojections coated with vaccine for delivery into the skin. Microarray patches (MAPs) offer the possibility of improved vaccine thermostability as well as the potential to be safer, more acceptable, easier to use, and more cost-effective for the administration of vaccines than injection by needle and syringe (N&S). Here, we report a phase I trial using the Vaxxas HD-MAP to deliver a monovalent influenza vaccine that was to the best of our knowledge the first clinical trial to evaluate the safety, tolerability, and immunogenicity of lower doses of influenza vaccine delivered by MAPs.
Methods and findings
HD-MAPs were coated with a monovalent, split inactivated influenza virus vaccine containing A/Singapore/GP1908/2015 H1N1 haemagglutinin (HA). Between February 2018 and March 2018, 60 healthy adults (age 18–35 years) in Melbourne, Australia were enrolled into part A of the study and vaccinated with either: HD-MAPs delivering 15 μg of A/Singapore/GP1908/2015 H1N1 HA antigen (A-Sing) to the volar forearm (FA); uncoated HD-MAPs; intramuscular (IM) injection of commercially available quadrivalent influenza vaccine (QIV) containing A/Singapore/GP1908/2015 H1N1 HA (15 μg/dose); or IM injection of H1N1 HA antigen (15 μg/dose). After 22 days’ follow-up and assessment of the safety data, a further 150 healthy adults were enrolled and randomly assigned to 1 of 9 treatment groups. Participants (20 per group) were vaccinated with HD-MAPs delivering doses of 15, 10, 5, 2.5, or 0 μg of HA to the FA or 15 μg HA to the upper arm (UA), or IM injection of QIV. The primary objectives of the study were safety and tolerability. Secondary objectives were to assess the immunogenicity of the influenza vaccine delivered by HD-MAP. Primary and secondary objectives were assessed for up to 60 days post-vaccination. Clinical staff and participants were blind as to which HD-MAP treatment was administered and to administration of IM-QIV-15 or IM-A/Sing-15. All laboratory investigators were blind to treatment and participant allocation. Two further groups in part B (5 participants per group), not included in the main safety and immunological analysis, received HD-MAPs delivering 15 μg HA or uncoated HD-MAPs applied to the forearm. Biopsies were taken on days 1 and 4 for analysis of the cellular composition from the HD-MAP application sites.
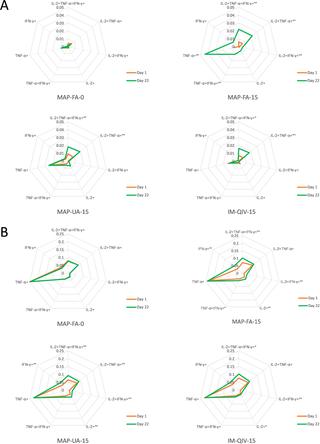
The vaccine coated onto HD-MAPs was antigenically stable when stored at 40°C for at least 12 months. HD-MAP vaccination was safe and well tolerated; any systemic or local adverse events (AEs) were mild or moderate. Observed systemic AEs were mostly headache or myalgia, and local AEs were application-site reactions, usually erythema. HD-MAP administration of 2.5 μg HA induced haemagglutination inhibition (HAI) and microneutralisation (MN) titres that were not significantly different to those induced by 15 μg HA injected IM (IM-QIV-15). HD-MAP delivery resulted in enhanced humoral responses compared with IM injection with higher HAI geometric mean titres (GMTs) at day 8 in the MAP-UA-15 (GMT 242.5, 95% CI 133.2–441.5), MAP-FA-15 (GMT 218.6, 95% CI 111.9–427.0), and MAP-FA-10 (GMT 437.1, 95% CI 254.3–751.3) groups compared with IM-QIV-15 (GMT 82.8, 95% CI 42.4–161.8), p = 0.02, p = 0.04, p < 0.001 for MAP-UA-15, MAP-FA-15, and MAP-FA-10, respectively. Higher titres were also observed at day 22 in the MAP-FA-10 (GMT 485.0, 95% CI 301.5–780.2, p = 0.001) and MAP-UA-15 (367.6, 95% CI 197.9–682.7, p = 0.02) groups compared with the IM-QIV-15 group (GMT 139.3, 95% CI 79.3–244.5). Results from a panel of exploratory immunoassays (antibody-dependent cellular cytotoxicity, CD4+ T-cell cytokine production, memory B cell (MBC) activation, and recognition of non-vaccine strains) indicated that, overall, Vaxxas HD-MAP delivery induced immune responses that were similar to, or higher than, those induced by IM injection of QIV. The small group sizes and use of a monovalent influenza vaccine were limitations of the study.
Conclusions
Influenza vaccine coated onto the HD-MAP was stable stored at temperatures up to 40°C. Vaccination using the HD-MAP was safe and well tolerated and resulted in immune responses that were similar to or significantly enhanced compared with IM injection. Using the HD-MAP, a 2.5 μg dose (1/6 of the standard dose) induced HAI and MN titres similar to those induced by 15 μg HA injected IM.
Trial registration
Australian New Zealand Clinical Trials Registry (ANZCTR.org.au), trial ID 108 ACTRN12618000112268/U1111-1207-3550.
中文翻译:

使用高密度微阵列贴片接种流感疫苗的安全性,耐受性和免疫原性:来自随机对照I期临床试验的结果。
背景
Vaxxas高密度微阵列贴片(HD-MAP)由高密度的微突出物组成,这些微突出物涂有疫苗,可以递送到皮肤中。微阵列贴片(MAP)提供了提高疫苗热稳定性的可能性,并且具有比通过针头和注射器(N&S)注射更安全,更容易接受,更易于使用且更具成本效益的疫苗管理潜力。在这里,我们报告了使用Vaxxas HD-MAP交付单价流感疫苗的第一阶段试验,据我们所知,这是第一项评估MAPs较低剂量流感疫苗安全性,耐受性和免疫原性的临床试验。
方法和发现
HD-MAPs用含有A / Singapore / GP1908 / 2015 H1N1血凝素(HA)的单价分裂灭活流感病毒疫苗包被。在2018年2月至2018年3月之间,澳大利亚墨尔本的60名健康成年人(18-35岁)被纳入研究的A部分并接种了以下疫苗:HD-MAPs递送15μgA / Singapore / GP1908 / 2015 H1N1 HA掌前臂(FA)的抗原(A-Sing); 未经涂层的HD-MAP;肌内(IM)注射含有A / Singapore / GP1908 / 2015 H1N1 HA(15μg/剂量)的市售四价流感疫苗(QIV); 或IM注射H1N1 HA抗原(15μg/剂量)。在22天的随访和安全性数据评估后,又招募了150名健康成人,并将其随机分配到9个治疗组中的1个中。参加者(每组20人)接种了HD-MAP,接种剂量为15 向FA注入10、5、2.5或0μgHA或向上臂(UA)注入15μgHA,或IM注射QIV。该研究的主要目标是安全性和耐受性。次要目标是评估HD-MAP提供的流感疫苗的免疫原性。疫苗接种后60天内评估了主要和次要目标。临床人员和参与者对采用哪种HD-MAP治疗以及IM-QIV-15或IM-A / Sing-15的治疗均无知。所有实验室研究人员对治疗和参与者分配均视而不见。B部分的其他两组(每组5名参与者)未包括在主要安全性和免疫学分析中,他们接受了HD-MAP递送15μgHA或未包被的HD-MAP应用于前臂。
当在40°C下保存至少12个月时,涂在HD-MAPs上的疫苗具有抗原稳定性。HD-MAP疫苗接种安全且耐受良好;任何全身或局部不良事件(AE)均为轻度或中度。观察到的全身AE主要是头痛或肌痛,局部AE是应用部位的反应,通常是红斑。HD-MAP施用2.5μgHA诱导的血凝抑制(HAI)和微中和(MN)滴度与15μgHA注射IM(IM-QIV-15)诱导的滴度没有显着差异。与第8天在MAP-UA-15(GMT 242.5,95%CI 133.2–441.5),MAP-FA-15(8)中的HAI几何平均滴度(GMT)较高的IM注射相比,HD-MAP递送导致增强的体液反应GMT 218.6、95%CI 111.9-427.0)和MAP-FA-10(GMT 437.1、95%CI 254.3-751.3)组与IM-QIV-15(GMT 82.8,对于MAP-UA-15,MAP-FA-15和MAP-FA-10,分别为p = 0.02,p = 0.04,p < 0.001。MAP-FA-10(GMT 485.0,95%CI 301.5-780.2,p = 0.001)和MAP-UA-15(367.6,95%CI 197.9-682.7,p = 0.02)在第22天也观察到更高的滴度。与IM-QIV-15组相比(GMT 139.3,95%CI 79.3–244.5)。一系列探索性免疫测定的结果(抗体依赖性细胞毒性,CD4 +T细胞细胞因子的产生,记忆B细胞(MBC)的激活以及对非疫苗株的识别)表明,总体而言,Vaxxas HD-MAP递送诱导的免疫反应与IM注射IM诱导的免疫反应相似或更高。 QIV。小组人数小和单价流感疫苗的使用是该研究的局限性。
结论
涂在HD-MAP上的流感疫苗可以稳定地储存在最高40°C的温度下。使用HD-MAP进行疫苗接种是安全且耐受性良好的,并且产生的免疫反应与IM注射疫苗相似或明显增强。使用HD-MAP,2.5μg剂量(标准剂量的1/6)诱导的HAI和MN滴度与15μgHA注射的IM诱导的滴度相似。
试用注册
澳大利亚新西兰临床试验注册中心(ANZCTR.org.au),试验ID 108 ACTRN12618000112268 / U1111-1207-3550。











































 京公网安备 11010802027423号
京公网安备 11010802027423号