Cell Death Discovery ( IF 6.1 ) Pub Date : 2020-03-17 , DOI: 10.1038/s41420-020-0249-4 Silvia von Karstedt 1, 2, 3 , Henning Walczak 2, 4, 5
|
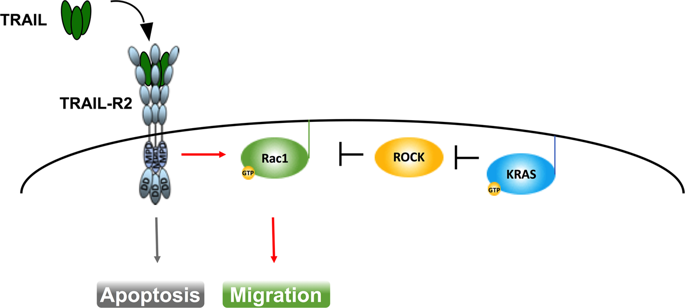
Twenty-one percent of all human cancers bear constitutively activating mutations in the proto-oncogene KRAS. This incidence is substantially higher in some of the most inherently therapy-resistant cancers including 30% of non-small cell lung cancers (NSCLC), 50% of colorectal cancers, and 95% of pancreatic ductal adenocarcinomas (PDAC). Importantly, survival of patients with KRAS-mutated PDAC and NSCLC has not significantly improved since the 1970s highlighting an urgent need to re-examine how oncogenic KRAS influences cell death signaling outputs. Interestingly, cancers expressing oncogenic KRAS manage to escape antitumor immunity via upregulation of programmed cell death 1 ligand 1 (PD-L1). Recently, the development of next-generation KRASG12C-selective inhibitors has shown therapeutic efficacy by triggering antitumor immunity. Yet, clinical trials testing immune checkpoint blockade in KRAS-mutated cancers have yielded disappointing results suggesting other, additional means endow these tumors with the capacity to escape immune recognition. Intriguingly, oncogenic KRAS reprograms regulated cell death pathways triggered by death receptors of the tumor necrosis factor (TNF) receptor superfamily. Perverting the course of their intended function, KRAS-mutated cancers use endogenous TNF-related apoptosis-inducing ligand (TRAIL) and its receptor(s) to promote tumor growth and metastases. Yet, endogenous TRAIL–TRAIL-receptor signaling can be therapeutically targeted and, excitingly, this may not only counteract oncogenic KRAS-driven cancer cell migration, invasion, and metastasis, but also the immunosuppressive reprogramming of the tumor microenvironment it causes. Here, we provide a concise summary of the current literature on oncogenic KRAS-mediated reprogramming of cell death signaling and antitumor immunity with the aim to open novel perspectives on combinatorial treatment strategies involving death receptor targeting.
中文翻译:

意外的命运转变:针对 KRAS 驱动的癌症中的 TRAIL-R 进行靶向治疗
21% 的人类癌症中存在原癌基因KRAS的组成性激活突变。在一些本质上对治疗耐药的癌症中,这种发生率要高得多,包括 30% 的非小细胞肺癌 (NSCLC)、50% 的结直肠癌和 95% 的胰腺导管腺癌 (PDAC)。重要的是,自 20 世纪 70 年代以来,KRAS 突变 PDAC 和 NSCLC 患者的生存率并未显着改善,这凸显了重新检查致癌 KRAS 如何影响细胞死亡信号输出的迫切需要。有趣的是,表达致癌 KRAS 的癌症通过上调程序性细胞死亡 1 配体 1 (PD-L1) 设法逃避抗肿瘤免疫。最近,下一代 KRAS G12C选择性抑制剂的开发已显示出通过触发抗肿瘤免疫的治疗功效。然而,在 KRAS 突变癌症中测试免疫检查点阻断的临床试验产生了令人失望的结果,表明其他额外手段赋予这些肿瘤逃避免疫识别的能力。有趣的是,致癌 KRAS 重新编程由肿瘤坏死因子 (TNF) 受体超家族的死亡受体触发的调节细胞死亡途径。 KRAS 突变的癌症利用内源性 TNF 相关凋亡诱导配体 (TRAIL) 及其受体来促进肿瘤生长和转移,从而破坏了其预期功能。然而,内源性 TRAIL-TRAIL 受体信号传导可以作为治疗靶点,令人兴奋的是,这不仅可以抵消致癌 KRAS 驱动的癌细胞迁移、侵袭和转移,还可以抵消其引起的肿瘤微环境的免疫抑制重编程。 在这里,我们对致癌 KRAS 介导的细胞死亡信号重编程和抗肿瘤免疫的当前文献进行了简要总结,旨在为涉及死亡受体靶向的组合治疗策略开辟新的视角。











































 京公网安备 11010802027423号
京公网安备 11010802027423号