Modern Pathology ( IF 7.1 ) Pub Date : 2020-03-16 , DOI: 10.1038/s41379-020-0519-y Xiaoling Wang 1 , Xiaodong Teng 1 , Wei Ding 1 , Ke Sun 1 , Bo Wang 1
|
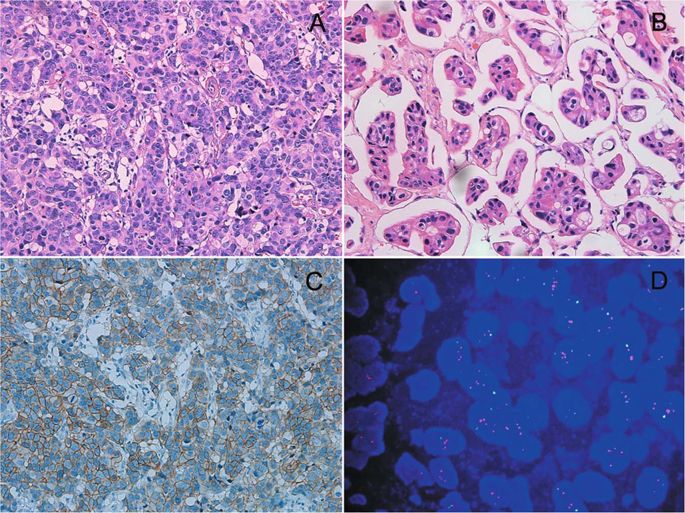
The American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) have recently issued updated guidelines on human epidermal growth factor receptor 2 (HER2) testing by fluorescence in situ hybridization (FISH) in invasive breast cancers. Cases with a HER2/chromosome enumeration probe 17 (CEP17) ratio of ≥2.0 but an average HER2 copy number of <4.0 signals per cell (ISH group 2) are no longer automatically classified as ISH positive. Herein, 30 cases in ISH group 2 were collected. Another 100 patients with a HER2/CEP17 ratio <2.0 and <4.0 HER2 signals per cell (ISH group 5) and 100 patients with a HER2/CEP17 ratio of ≥2.0 and an average HER2 copy number of ≥4.0 signals per cell (ISH group 1) were also recruited for comparison. According to the 2018 ASCO/CAP guidelines, all the cases in ISH group 2 were categorized as HER2 negative. The clinicopathological characteristics of the patients in ISH group 2 were intermediate between ISH group 1 and group 5. Survival analyses revealed that there was no significant disease-free survival (DFS) and overall survival (OS) difference between patients with or without targeted therapy in ISH group 2, as well as between patients with targeted therapy in ISH group 1 and patients in ISH group 2. Patients without targeted therapy in ISH group 2 had a significantly worse OS than patients with targeted therapy in ISH group 1 and patients in ISH group 5. In conclusion, patients in ISH group 2 represent a biologically heterogeneous subset, which are different from those in ISH group 1 and 5. A larger cohort of patients in ISH group 2 should be included for future researches to define the efficacy of HER2-targeted therapy.
中文翻译:

一项针对 HER2/CEP17 比率≥2.0 但平均 HER2 拷贝数 <4.0 每个细胞信号的 30 例乳腺癌病例的临床病理学研究。
美国临床肿瘤学会 (ASCO)/美国病理学家学会 (CAP) 最近发布了关于在浸润性乳腺癌中通过荧光原位杂交 (FISH) 检测人表皮生长因子受体 2 (HER2) 的最新指南。HER2/染色体计数探针 17 (CEP17) 比率≥2.0 但每个细胞的平均 HER2 拷贝数 <4.0 个信号(ISH 组 2)的病例不再自动归类为 ISH 阳性。此处收集ISH第2组30例。另外 100 名 HER2/CEP17 比率 <2.0 且每个细胞 <4.0 HER2 信号的患者(ISH 组 5)和 100 名 HER2/CEP17 比率≥2.0 且平均 HER2 拷贝数≥4.0 每个细胞信号的患者(ISH 组1)也被招募进行比较。根据 2018 年 ASCO/CAP 指南,ISH 组 2 中的所有病例都被归类为 HER2 阴性。ISH 组 2 患者的临床病理特征介于 ISH 组 1 和组 5 之间。生存分析显示,在接受或不接受靶向治疗的患者中,无病生存期 (DFS) 和总生存期 (OS) 无显着差异ISH 组 2,以及 ISH 组 1 中接受靶向治疗的患者和 ISH 组 2 中的患者之间。ISH 组 2 中未接受靶向治疗的患者的 OS 显着低于 ISH 组 1 中接受靶向治疗的患者和 ISH 组中的患者5. 总之,ISH 组 2 中的患者代表了一个生物学异质性子集,与 ISH 组 1 和 5 中的患者不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号