Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2-17 years: a randomised, placebo-controlled, phase 2 trial.
The Lancet ( IF 98.4 ) Pub Date : 2020-03-17 , DOI: 10.1016/s0140-6736(20)30556-0 Vianney Tricou 1 , Xavier Sáez-Llorens 2 , Delia Yu 3 , Luis Rivera 4 , José Jimeno 5 , Ana Cecilia Villarreal 5 , Epiphany Dato 3 , Onix Saldaña de Suman 5 , Nathali Montenegro 5 , Rodrigo DeAntonio 6 , Sonia Mazara 4 , Maria Vargas 4 , Debbie Mendoza 7 , Martina Rauscher 1 , Manja Brose 1 , Inge Lefevre 1 , Suely Tuboi 8 , Astrid Borkowski 1 , Derek Wallace 9
The Lancet ( IF 98.4 ) Pub Date : 2020-03-17 , DOI: 10.1016/s0140-6736(20)30556-0 Vianney Tricou 1 , Xavier Sáez-Llorens 2 , Delia Yu 3 , Luis Rivera 4 , José Jimeno 5 , Ana Cecilia Villarreal 5 , Epiphany Dato 3 , Onix Saldaña de Suman 5 , Nathali Montenegro 5 , Rodrigo DeAntonio 6 , Sonia Mazara 4 , Maria Vargas 4 , Debbie Mendoza 7 , Martina Rauscher 1 , Manja Brose 1 , Inge Lefevre 1 , Suely Tuboi 8 , Astrid Borkowski 1 , Derek Wallace 9
Affiliation
|
BACKGROUND
An unmet clinical need remains for an effective tetravalent dengue vaccine suitable for all age groups, regardless of serostatus. We assessed the immunogenicity and safety of three different dose schedules of a tetravalent dengue vaccine (TAK-003) over a 48-month period in children living in dengue-endemic countries.
METHODS
We did a large, phase 2, double-blind, placebo-controlled trial at three sites in the Dominican Republic, Panama, and the Philippines. Healthy participants aged 2-17 years were randomly assigned 1:2:5:1 using an interactive web response system with stratification by age to receive either a two-dose primary series (days 1 and 91), one primary dose (day 1), one primary dose plus booster (days 1 and 365), or placebo. Participants and relevant study personnel were masked to the random assignment until completion of the study at month 48. To maintain masking, TAK-003 recipients were administered placebo doses when appropriate. The primary objective was assessment of neutralising geometric mean titres for each serotype to month 48 assessed in the per-protocol immunogenicity subset. Secondary safety endpoints included proportions of participants with serious adverse events and symptomatic virologically confirmed dengue. This study is registered with ClinicalTrials.gov, NCT02302066.
FINDINGS
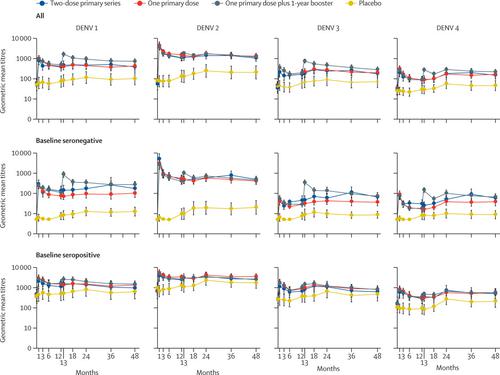
Between Dec 5, 2014, and Feb 13, 2015, 1800 children were randomly assigned to the following groups: two-dose primary series (n=201), one primary dose (n=398), one primary dose plus 1-year booster (n=1002), and placebo (n=199). Of them, 1479 (82%) participants completed the 48-month study. Immunogenicity endpoints were assessed in 562 participants enrolled in the immunogenicity subset, of whom 509 were included in the per-protocol subset. At month 48, antibody titres remained elevated in all TAK-003 groups compared with placebo, irrespective of baseline serostatus. At month 48, geometric mean titres were 378 (95% CI 226-632) in two-dose, 421 (285-622) in one-dose, 719 (538-960) in one-dose plus 1-year booster, and 100 (50-201) in placebo recipients against DENV 1; 1052 (732-1511), 1319 (970-1794), 1200 (927-1553), and 208 (99-437) against DENV 2; 183 (113-298), 201 (135-298), 288 (211-392), and 71 (37-139) against DENV 3; and 152 (97-239), 164 (114-236), 219 (165-290), and 46 (26-82) against DENV 4; and tetravalent seropositivity rate was 89% (79-96), 86% (80-92), 97% (93-99), and 60% (47-72), respectively. Virologically confirmed dengue was recorded in 37 (2%) TAK-003 and 13 (7%) placebo participants, with a relative risk of 0·35 (0·19-0·65). No vaccine-related serious adverse events or severe dengue virus disease were reported.
INTERPRETATION
TAK-003 elicited antibody responses against all four serotypes, which persisted to 48 months post-vaccination, regardless of baseline serostatus. No important safety risks were identified. We observed a long-term reduction in risk of symptomatic dengue virus disease in vaccinees. Results from this study provide a long-term safety database and support assessment of the vaccine in the ongoing phase 3 efficacy study.
FUNDING
Takeda Vaccines.
中文翻译:

四价登革热疫苗在2-17岁儿童中的安全性和免疫原性:一项随机,安慰剂对照的2期试验。
背景技术仍然需要满足适用于所有年龄段而不论血清状况如何的有效的四价登革热疫苗的临床需求。我们评估了居住在登革热流行国家的儿童在48个月内三种不同剂量方案的四价登革热疫苗(TAK-003)的免疫原性和安全性。方法我们在多米尼加共和国,巴拿马和菲律宾的三个地点进行了一项大型的2期,双盲,安慰剂对照试验。使用互动式网络反应系统按年龄分层将2-17岁的健康参与者随机分配为1:2:5:1,以接受两剂一次系列(第1和91天),一剂一次(第1天) ,一剂主要剂量加加强剂量(第1和365天)或安慰剂。参与者和相关研究人员被屏蔽后随机分配,直到研究在第48个月完成。为了维持屏蔽作用,在适当的时候给TAK-003接受者服用安慰剂。主要目的是评估在每种方案的免疫原性亚组中评估的每种血清型至48个月的几何平均滴度。次要安全性终点指标包括严重不良事件和有症状的病毒学确认登革热参与者的比例。该研究已在ClinicalTrials.gov注册,NCT02302066。结果在2014年12月5日至2015年2月13日之间,将1800名儿童随机分为以下几组:两剂一次剂量系列(n = 201),一剂一次剂量(n = 398),一剂一次剂量加1年增强剂(n = 1002)和安慰剂(n = 199)。在他们当中 1479(82%)名参与者完成了48个月的研究。免疫原性亚组中的562名参与者对免疫原性终点进行了评估,其中每方案亚组中包括509名参与者。在第48个月时,与基线血清状况无关,与安慰剂相比,所有TAK-003组的抗体滴度仍然升高。在第48个月,两剂的几何平均滴度为378(95%CI 226-632),一剂为421(285-622),一剂为1年加效期的719(538-960),以及接受DENV 1的安慰剂接受者100(50-201);针对DENV 2的1052(732-1511),1319(970-1794),1200(927-1553)和208(99-437);对抗DENV 3的183(113-298),201(135-298),288(211-392)和71(37-139);和152(97-239),164(114-236),219(165-290)和46(26-82)对抗DENV 4;四价血清阳性率为89%(79-96),86%(80-92),97%(93-99),和60%(47-72)。经病毒学确认的登革热在37名(2%)TAK-003和13名(7%)安慰剂参与者中记录,相对风险为0·35(0·19-0·65)。没有报告与疫苗有关的严重不良事件或严重的登革热病毒病。解释TAK-003引发针对所有四种血清型的抗体应答,无论基线血清状况如何,这种应答均持续至疫苗接种后48个月。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并支持正在进行的3期功效研究中的疫苗评估。资助武田疫苗。相对风险为0·35(0·19-0·65)。没有报告疫苗相关的严重不良事件或严重的登革热病毒病。解释TAK-003引发针对所有四种血清型的抗体应答,无论基线血清状况如何,这种应答均持续至疫苗接种后48个月。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并支持正在进行的3期功效研究中的疫苗评估。资助武田疫苗。相对风险为0·35(0·19-0·65)。没有报告与疫苗有关的严重不良事件或严重的登革热病毒病。解释TAK-003引发针对所有四种血清型的抗体应答,无论基线血清状况如何,这种应答均持续至疫苗接种后48个月。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并支持正在进行的3期功效研究中的疫苗评估。资助武田疫苗。不论基线血清状况如何。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并支持正在进行的3期功效研究中的疫苗评估。资助武田疫苗。不论基线血清状况如何。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并在正在进行的3期功效研究中支持了疫苗的评估。资助武田疫苗。
更新日期:2020-04-22
中文翻译:

四价登革热疫苗在2-17岁儿童中的安全性和免疫原性:一项随机,安慰剂对照的2期试验。
背景技术仍然需要满足适用于所有年龄段而不论血清状况如何的有效的四价登革热疫苗的临床需求。我们评估了居住在登革热流行国家的儿童在48个月内三种不同剂量方案的四价登革热疫苗(TAK-003)的免疫原性和安全性。方法我们在多米尼加共和国,巴拿马和菲律宾的三个地点进行了一项大型的2期,双盲,安慰剂对照试验。使用互动式网络反应系统按年龄分层将2-17岁的健康参与者随机分配为1:2:5:1,以接受两剂一次系列(第1和91天),一剂一次(第1天) ,一剂主要剂量加加强剂量(第1和365天)或安慰剂。参与者和相关研究人员被屏蔽后随机分配,直到研究在第48个月完成。为了维持屏蔽作用,在适当的时候给TAK-003接受者服用安慰剂。主要目的是评估在每种方案的免疫原性亚组中评估的每种血清型至48个月的几何平均滴度。次要安全性终点指标包括严重不良事件和有症状的病毒学确认登革热参与者的比例。该研究已在ClinicalTrials.gov注册,NCT02302066。结果在2014年12月5日至2015年2月13日之间,将1800名儿童随机分为以下几组:两剂一次剂量系列(n = 201),一剂一次剂量(n = 398),一剂一次剂量加1年增强剂(n = 1002)和安慰剂(n = 199)。在他们当中 1479(82%)名参与者完成了48个月的研究。免疫原性亚组中的562名参与者对免疫原性终点进行了评估,其中每方案亚组中包括509名参与者。在第48个月时,与基线血清状况无关,与安慰剂相比,所有TAK-003组的抗体滴度仍然升高。在第48个月,两剂的几何平均滴度为378(95%CI 226-632),一剂为421(285-622),一剂为1年加效期的719(538-960),以及接受DENV 1的安慰剂接受者100(50-201);针对DENV 2的1052(732-1511),1319(970-1794),1200(927-1553)和208(99-437);对抗DENV 3的183(113-298),201(135-298),288(211-392)和71(37-139);和152(97-239),164(114-236),219(165-290)和46(26-82)对抗DENV 4;四价血清阳性率为89%(79-96),86%(80-92),97%(93-99),和60%(47-72)。经病毒学确认的登革热在37名(2%)TAK-003和13名(7%)安慰剂参与者中记录,相对风险为0·35(0·19-0·65)。没有报告与疫苗有关的严重不良事件或严重的登革热病毒病。解释TAK-003引发针对所有四种血清型的抗体应答,无论基线血清状况如何,这种应答均持续至疫苗接种后48个月。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并支持正在进行的3期功效研究中的疫苗评估。资助武田疫苗。相对风险为0·35(0·19-0·65)。没有报告疫苗相关的严重不良事件或严重的登革热病毒病。解释TAK-003引发针对所有四种血清型的抗体应答,无论基线血清状况如何,这种应答均持续至疫苗接种后48个月。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并支持正在进行的3期功效研究中的疫苗评估。资助武田疫苗。相对风险为0·35(0·19-0·65)。没有报告与疫苗有关的严重不良事件或严重的登革热病毒病。解释TAK-003引发针对所有四种血清型的抗体应答,无论基线血清状况如何,这种应答均持续至疫苗接种后48个月。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并支持正在进行的3期功效研究中的疫苗评估。资助武田疫苗。不论基线血清状况如何。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并支持正在进行的3期功效研究中的疫苗评估。资助武田疫苗。不论基线血清状况如何。没有发现重要的安全风险。我们观察到疫苗中有症状的登革热病毒疾病的风险长期降低。这项研究的结果提供了长期的安全性数据库,并在正在进行的3期功效研究中支持了疫苗的评估。资助武田疫苗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号